Promising Interim Results for B7-H3 Antibody-Drug Conjugate by Duality Biologics and BioNTech at 2024 ESMO Asia Congress
Duality Biologics announced initial findings from a global Phase 1/2a clinical trial (NCT05914116, CTR20232835) assessing BNT324/DB-1311, an experimental next-generation antibody-drug conjugate that targets the transmembrane glycoprotein B7-H3. These results were shared during an oral session at the 2024 European Society of Medical Oncology Asia Annual Meeting held in Singapore. The findings demonstrated promising antitumor effects and a manageable safety profile in patients with locally advanced or metastatic solid tumors who have undergone extensive prior treatment. BNT324/DB-1311 is being jointly developed by BioNTech SE and DualityBio.
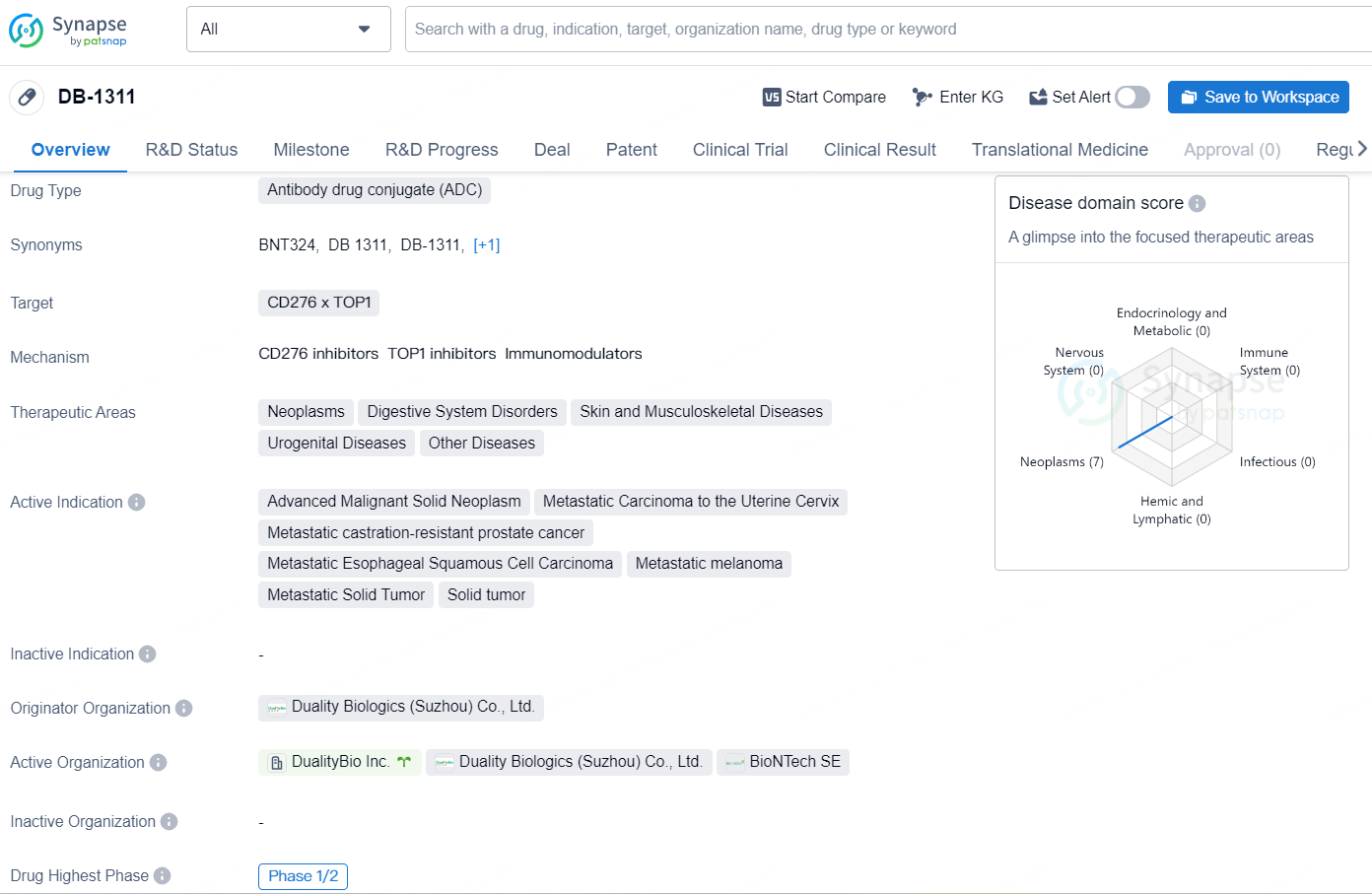
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The ongoing Phase 1/2a trial involved an analysis of 277 participants with various solid tumors such as small cell lung cancer (SCLC), non-small cell lung cancer (NSCLC), castration-resistant prostate cancer (CRPC), and squamous cell carcinoma of the head and neck (SCCHN). Approximately 75% of the participants had an Eastern Cooperative Oncology Group (ECOG) performance status of 1, and about 61% had received two or more lines of prior therapy. The primary goals of this trial are to assess safety and the objective response rate (ORR) as evaluated by investigators. Secondary objectives include duration of response (DoR), disease control rate (DCR), progression-free survival (PFS), and overall survival (OS), among others. The results indicated the following:
Among the 238 evaluable patients who had at least one post-baseline tumor assessment, the overall unadjusted ORR was 32.4% with a DCR of 82.4%. In the SCLC cohort (n=73), the unadjusted ORR reached 56.2%, while the DCR was 89.0%. The majority of SCLC patients received doses of 6 mg/kg and 9 mg/kg of BNT324/DB-1311, showing no significant difference in uORR between these two dosing regimens (54.5% vs. 58.8%). Particularly, within the 9 mg/kg dosing, SCLC patients who had previously undergone immunotherapy but not treated with topoisomerase I inhibitors exhibited an uORR of 70.4%.
For NSCLC patients, the majority had non-squamous histology (n=41) presenting an unadjusted ORR of 22.0%, whereas those with squamous NSCLC (n=25) had an ORR of 16.0%. In the CRPC subgroup (n=32), BNT324/DB-1311 illustrated early signs of antitumor activity, showing an unadjusted ORR of 28.0% and a DCR of 92.0%. The median rPFS was recorded at 7.2 months, with rPFS data still maturing at the time of analysis, and a 6-month rPFS rate of 94.7% was noted.
Among other tumor types, including cervical cancer (n=4), hepatocellular carcinoma (n=12), head and neck squamous carcinoma (n=3), and melanoma (n=11), BNT324/DB-1311 also demonstrated antitumor effects with respective uORRs of 75.0%, 25.0%, 100.0%, and 36.4%.
The safety profile of BNT324/DB-1311 was found to be manageable across all assessed patients and tumor types (n=277). Common treatment-related adverse events (TRAEs) included nausea, decreased neutrophil count, anemia, reduction in white blood cell count, decreased appetite, and reduced platelet count.
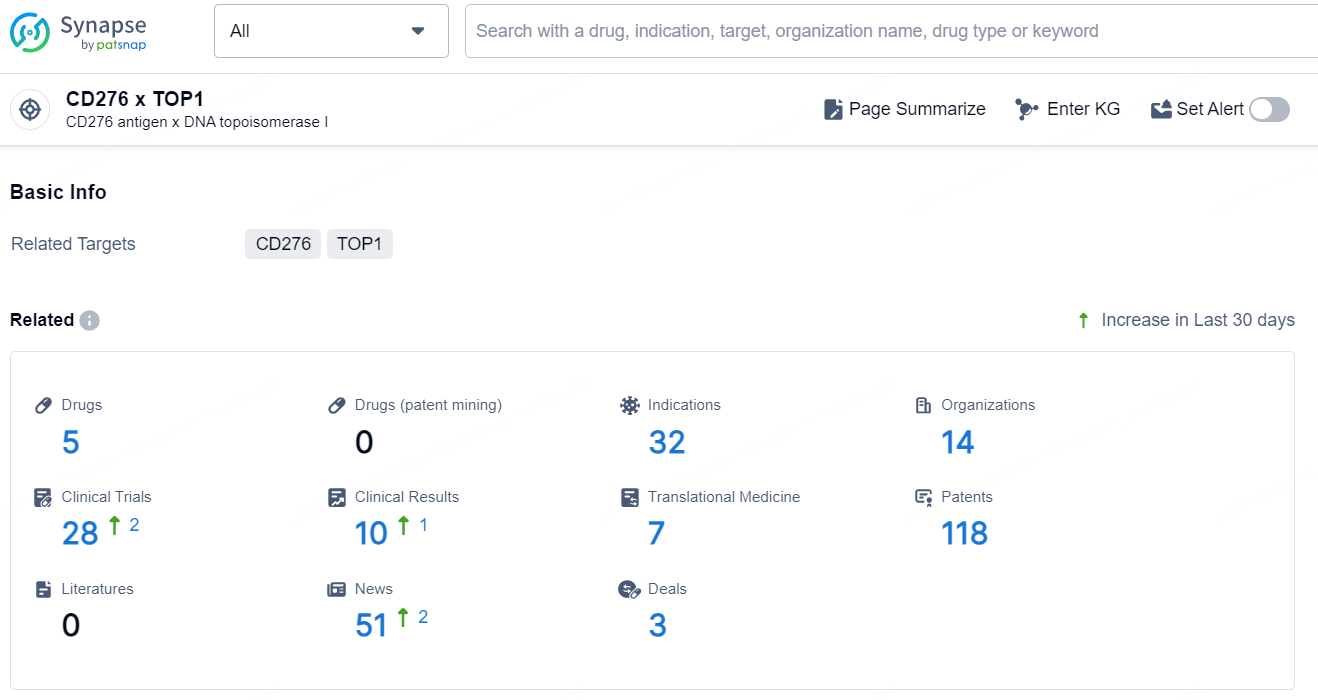
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of December 10, 2024, there are 5 investigational drug for the CD276 x TOP1 targets, including 32 indications, 14 R&D institutions involved, with related clinical trials reaching 28, and as many as 118 patents.
DB-1311 is an antibody drug conjugate (ADC) that targets CD276 and TOP1, making it a promising therapeutic option for various neoplastic and urogenital diseases, as well as digestive system, skin, and musculoskeletal disorders.