Takeda Enhances Oncology Portfolio by Licensing Elritercept from Keros Therapeutics
Takeda (TSE:4502/NYSE:TAK) has revealed that it has signed an exclusive licensing deal with Keros Therapeutics, Inc. (Nasdaq: KROS) aimed at advancing the development, production, and global commercialization of elritercept, excluding mainland China, Hong Kong, and Macau.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
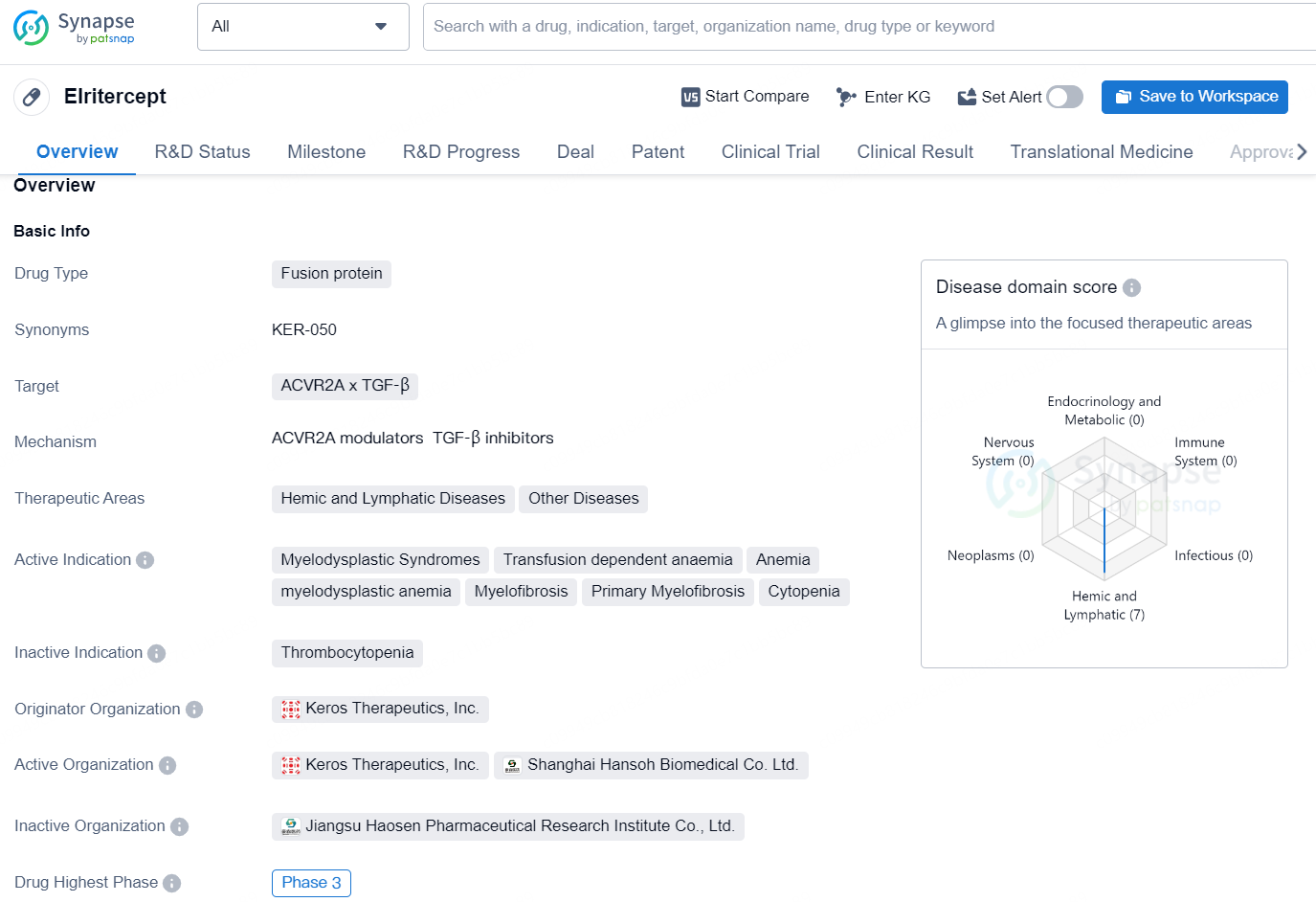
Elritercept is an advanced investigational activin inhibitor aimed at treating anemia linked with specific hematologic malignancies, such as myelodysplastic syndromes (MDS) and myelofibrosis (MF). The U.S. Food and Drug Administration (FDA) has awarded Fast Track designation to elritercept for its development in patients with very low-, low-, and intermediate-risk MDS. Both MDS and MF are marked by inadequate production of blood cells, often resulting in severe anemia that adversely affects patient health and life quality. Elritercept specifically affects activin A and B proteins, which are thought to be integral to diseases associated with anemia. Initial clinical trials have indicated that elritercept demonstrates promising therapeutic efficacy and an acceptable safety profile as a standalone treatment for patients with very low-, low-, and intermediate-risk MDS, as well as in conjunction with standard therapies for those with MF.
“Elritercept could significantly benefit patients suffering from blood cancers, which remains one of our principal strategic priorities,” stated Teresa Bitetti, President of the Global Oncology Business Unit at Takeda. “Incorporating elritercept strengthens our oncology portfolio and represents a potential avenue for growth for Takeda. I am eager to advance the innovative efforts initiated by the Keros Therapeutics team to provide this prospective treatment option to patients.”
Currently, elritercept is undergoing two Phase 2 clinical trials; one focuses on patients with very low-, low-, or intermediate-risk MDS, while the other involves patients with MF. The Phase 3 RENEW trial, which will assess elritercept in adult patients experiencing transfusion-dependent anemia with very low-, low-, or intermediate-risk MDS, is set to start enrolling participants shortly. Takeda intends to investigate elritercept within these cancer types across various patient populations and therapeutic lines.
“We are thrilled to collaborate with Takeda, whose extensive reach and expertise in oncology and hematology will facilitate the unlocking of elritercept’s potential for MDS and MF patients,” remarked Jasbir S. Seehra, Ph.D., Chair and CEO at Keros Therapeutics. “With its distinct mechanism of action targeting a wide array of pathways in blood cell production, elritercept has shown promise for patients who have not benefited from conventional therapies. This partnership aims to speed up the development of elritercept for those in need and provide new insights into these intricate hematologic disorders.”
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
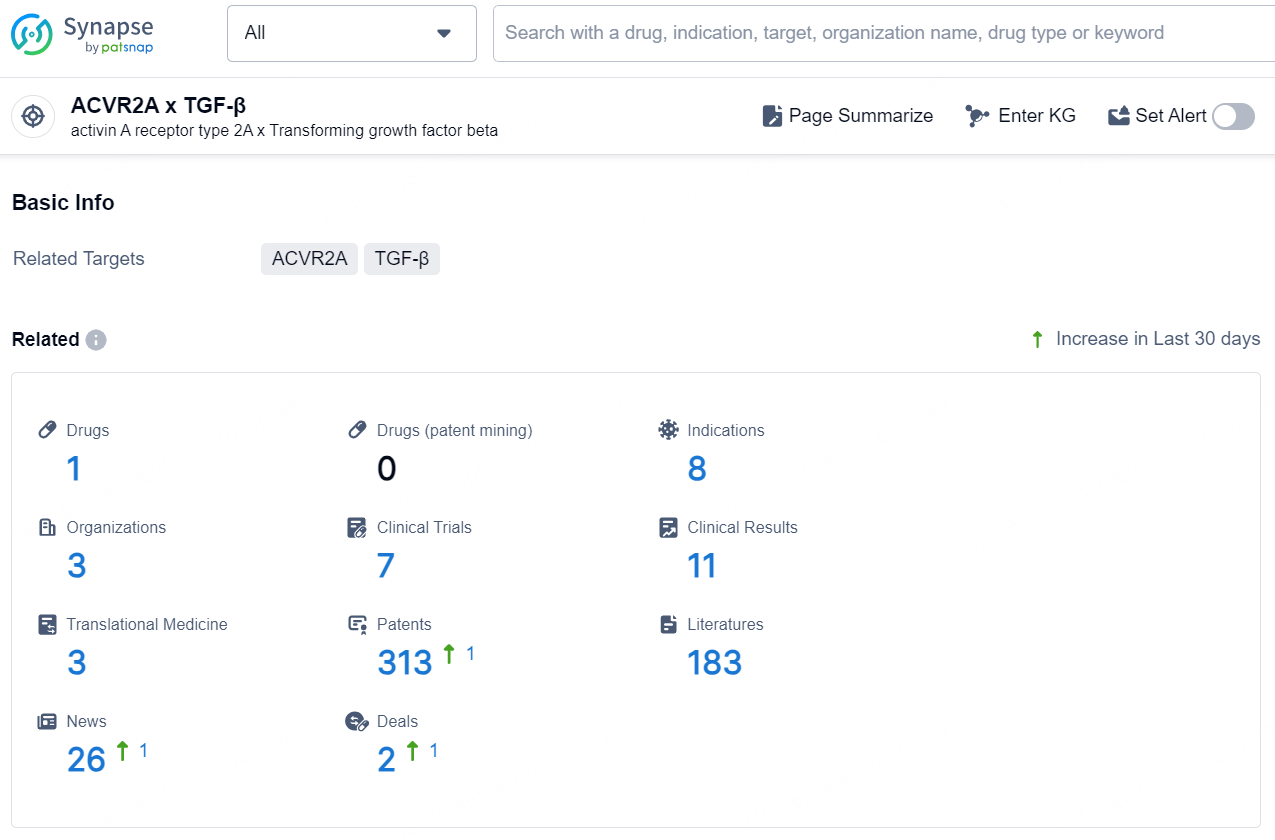
According to the data provided by the Synapse Chemical, As of December 6, 2024, there are 1 investigational drug for the ACVR2A x TGF-β target, including 8 indications, 3 R&D institutions involved, with related clinical trials reaching 7, and as many as 313 patents.
Elritercept is a fusion protein drug developed by Keros Therapeutics, Inc., targeting ACVR2A x TGF-β. It falls under the therapeutic areas of Hemic and Lymphatic Diseases, as well as Other Diseases. The drug is indicated for the treatment of Myelodysplastic Syndromes, Transfusion dependent anaemia, Anemia, myelodysplastic anemia, Myelofibrosis, Primary Myelofibrosis, and Cytopenia.