IMFINZI® Approved as First Immunotherapy for Limited-Stage Small Cell Lung Cancer in the US
AstraZeneca's IMFINZI® (durvalumab) has received approval in the United States for treating adult patients with limited-stage small cell lung cancer (LS-SCLC) who have not experienced disease progression after undergoing concurrent chemotherapy with platinum-based agents and radiation therapy.
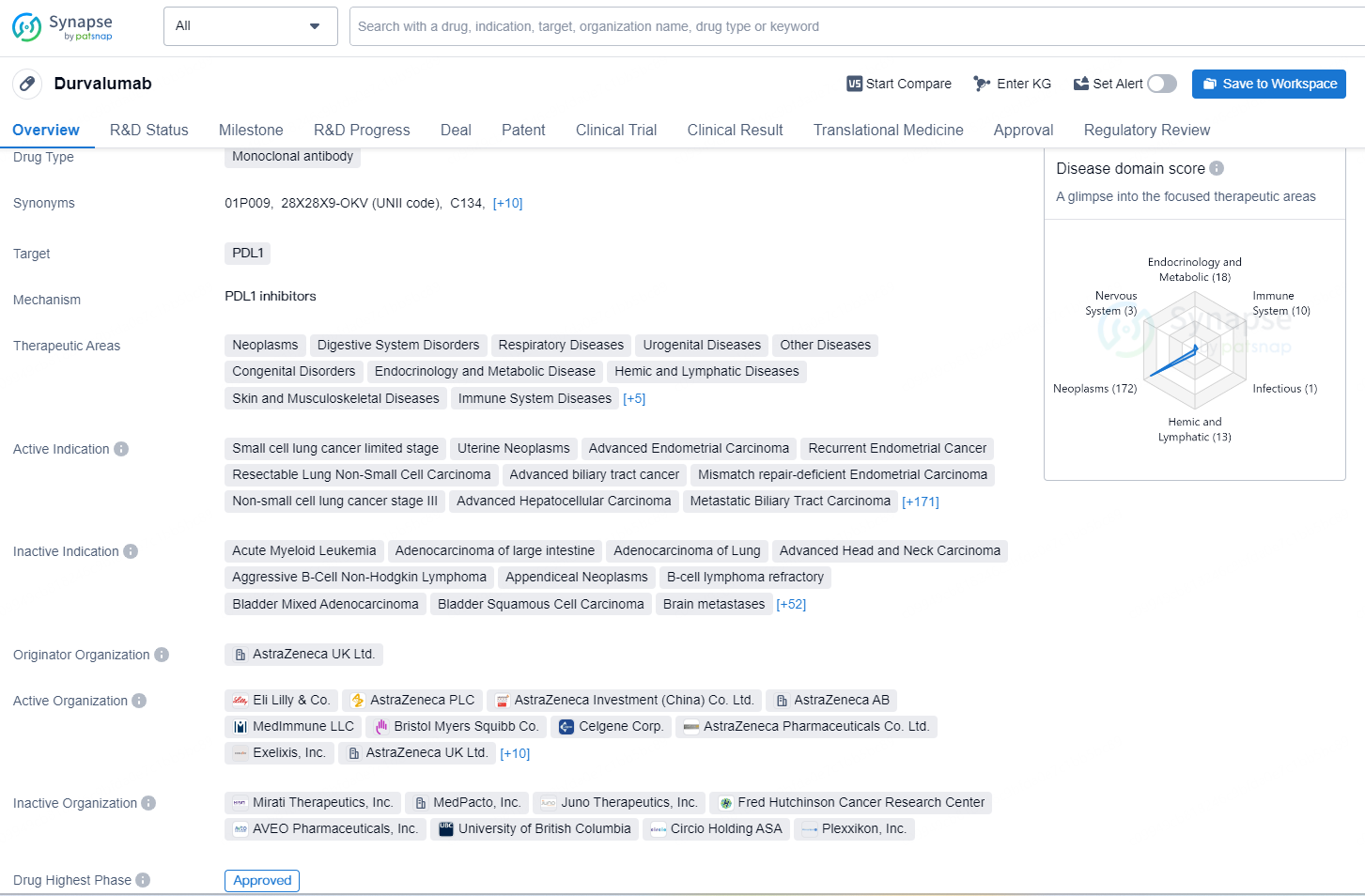
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The Food and Drug Administration (FDA) granted approval after obtaining both Priority Review and Breakthrough Therapy Designation. This decision was informed by outcomes from the ADRIATIC Phase III trial, which were presented at the Plenary Session of the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting and later published in the New England Journal of Medicine.
Small cell lung cancer (SCLC) is a notably aggressive type of lung cancer. Limited-stage SCLC (LS-SCLC) often shows rapid recurrence and progression, despite an initial positive response to conventional chemotherapy and radiotherapy. The survival outlook for LS-SCLC is particularly grim, with only 15-30% of patients surviving five years after their diagnosis.
Dr. Suresh Senan, a Professor of Clinical Experimental Radiotherapy at the Amsterdam University Medical Centers in the Netherlands and the trial’s international coordinating investigator, remarked, “Durvalumab stands as the sole systemic therapy following curative platinum-based chemoradiotherapy that has demonstrated an enhancement in survival for patients with this aggressive lung cancer. This represents the first significant advancement in four decades for this disease. The ADRIATIC trial demonstrated that 57% of patients were still alive three years post-durvalumab treatment, highlighting the transformative impact this therapy could have in clinical practice.”
Dave Fredrickson, AstraZeneca’s Executive Vice President for the Oncology Business Unit, commented, “The approval of IMFINZI marks a milestone for individuals with limited-stage small cell lung cancer, enabling them to access immunotherapy for the first time. The ADRIATIC trial indicated a median overall survival increase of 22.5 months, setting a new standard. IMFINZI is the sole immunotherapy approved for both limited- and extensive-stage small cell lung cancer, reflecting our dedication to enhancing survival rates.”
Dusty Donaldson, the Founder and Executive Director of LiveLung, stated, “This new treatment represents a significant development for individuals with limited-stage small cell lung cancer, a type of cancer characterized by a high recurrence rate. Traditionally, numerous clinical trials aimed at discovering new treatment options for this cancer have not demonstrated efficacy. We are thrilled that many more patients will now be able to access this immunotherapy, which has the potential to greatly improve patient outcomes.”
In the ADRIATIC trial, IMFINZI demonstrated a 27% reduction in mortality risk compared to placebo (with an overall survival [OS] hazard ratio [HR] of 0.73; 95% confidence interval [CI] 0.57-0.93; P=0.0104). The estimated median OS was 55.9 months for IMFINZI versus 33.4 months for the placebo group, with approximately 57% of IMFINZI recipients still alive at three years, compared to 48% for placebo.
Furthermore, IMFINZI reduced the risk of disease progression or death by 24% compared to placebo (reflected in a progression-free survival [PFS] HR of 0.76; 95% CI 0.61-0.95; P=0.0161). The median PFS was 16.6 months for IMFINZI against 9.2 months for placebo. About 46% of patients receiving IMFINZI had not experienced disease progression at the two-year mark, compared to 34% in the placebo group.
The safety profile of IMFINZI was found to be predominantly manageable and aligned with its established profile, with no new safety concerns identified.
Additionally, IMFINZI has received approval in Switzerland for this indication based on ADRIATIC results. Regulatory submissions are currently undergoing review in the European Union, Japan, and several other countries for this indication.
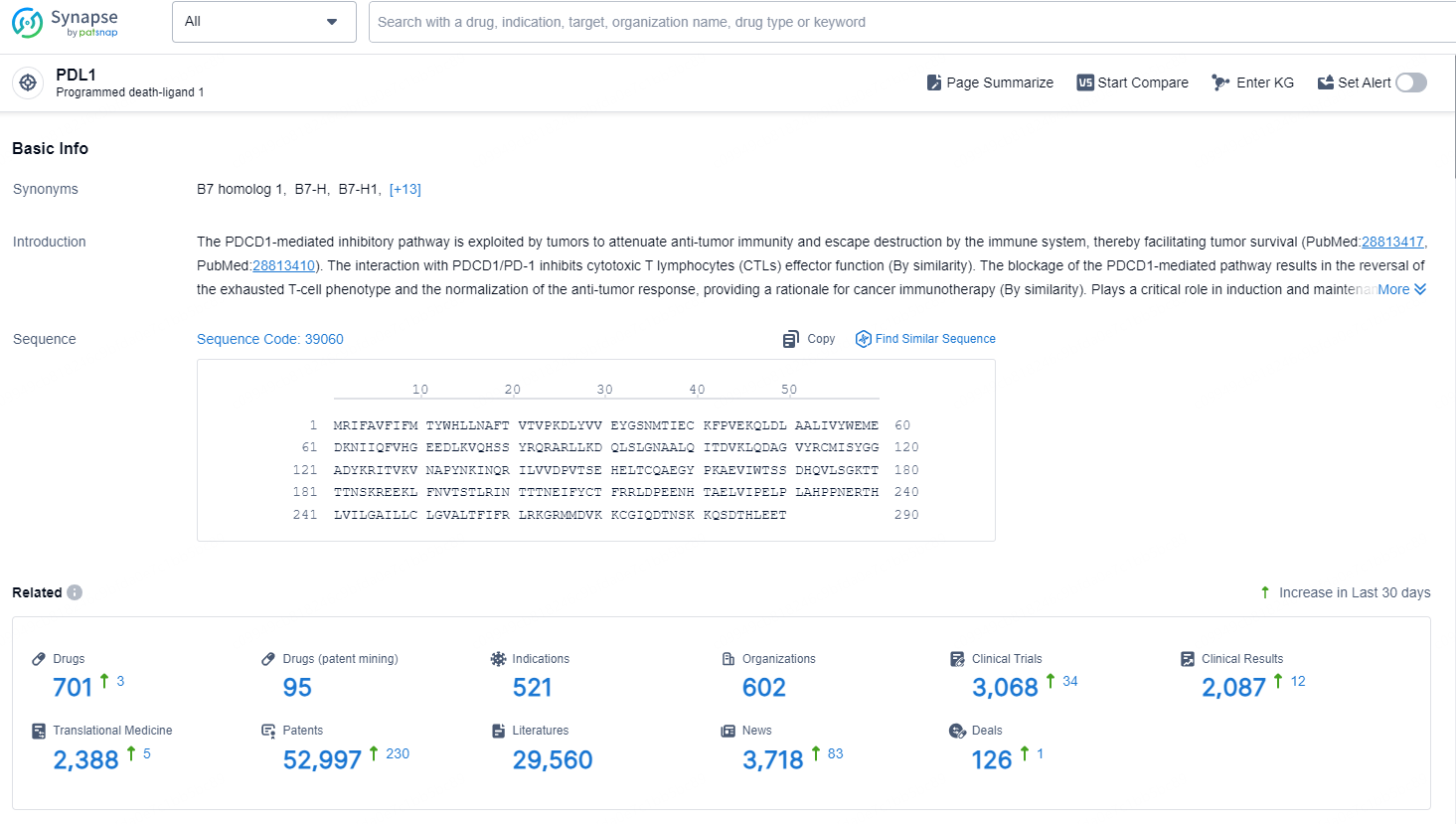
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of December 10, 2024, there are 701 investigational drugs for the PDL1 target, including 521 indications, 602 R&D institutions involved, with related clinical trials reaching 3068, and as many as 52997 patents.
Durvalumab is a drug that belongs to the class of monoclonal antibodies and functions as a programmed death-ligand 1 (PDL1) inhibitor. Its primary indication is for the treatment of Small Cell Lung Cancer. The drug has been approved for medical use in the United States as of December 4, 2018. The mechanism of action of Durvalumab involves binding to the PDL1 protein, which is a ligand for the PD-1 receptor on T-cells, thereby blocking the interaction between PD-1 and PDL1.