Merck and Ridgeback Begin Phase 3 Trial of LAGEVRIO™ for High-Risk COVID-19 Adults
Merck (NYSE: MRK), referred to as MSD outside of the U.S. and Canada, has announced the launch of the Phase 3 MOVe-NOW clinical trial aimed at assessing LAGEVRIOTM (molnupiravir), an experimental oral antiviral for COVID-19 treatment, specifically targeted at adults at significant risk of disease worsening. This global study is designed as a double-blind, placebo-controlled trial and is recruiting participants who are at least 18 years old, have tested positive for SARS-CoV-2, experienced COVID-19 symptoms for up to four days, and are not currently hospitalized. Moreover, eligible participants will be adults unable to take nirmatrelvir/ritonavir (NMV/r) due to potential drug interactions, allergies, prior adverse reactions, or lack of access.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The MOVe-NOW trial will utilize a novel formulation of LAGEVRIO that consists of two smaller 400-mg tablets per dose (resulting in a total of four tablets daily), contrasting with the existing regimen of four 200-mg capsules per dose (eight capsules per day). The new smaller tablets are not yet authorized for use in any nation. For further details regarding the study, please visit clinicaltrials.gov.
“COVID-19 is still a major contributor to hospitalizations and mortality globally, and ongoing research into LAGEVRIO could yield valuable insights into its potential role in preventing severe outcomes amid the current COVID-19 situation,” stated Dr. Paula Annunziato, senior vice president of infectious diseases and vaccines at Global Clinical Development, Merck Research Laboratories. “We maintain our belief that LAGEVRIO could serve as a critical option for individuals with risk factors such as advanced age, multiple comorbidities, and immunocompromising conditions, who are more susceptible to developing severe COVID-19, and for whom alternative antiviral therapies may not be viable due to possible drug-drug interactions.”
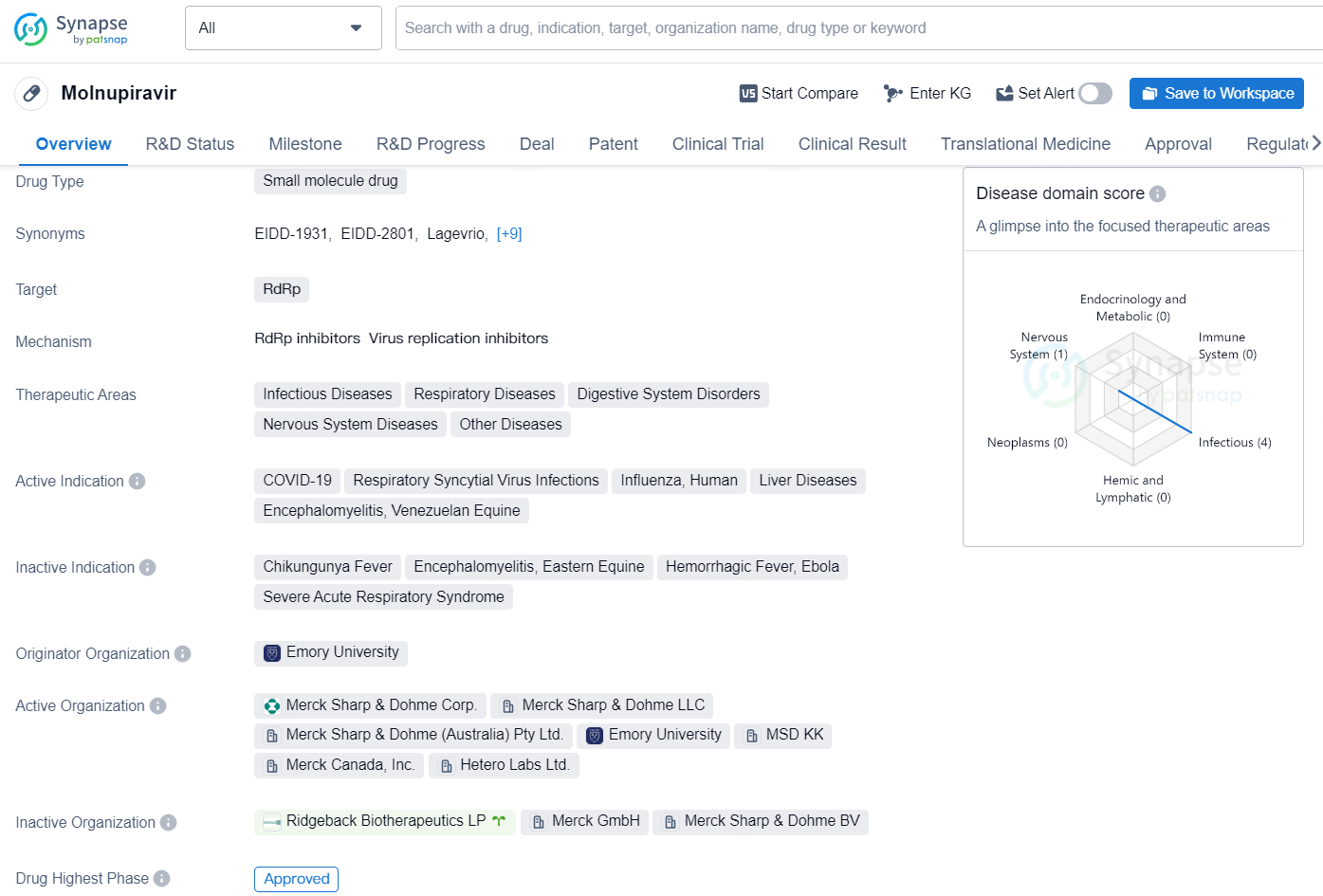
LAGEVRIO has been approved or authorized for use in various countries, such as Japan and Australia, and is available under an emergency use authorization in the United States for specific adults diagnosed with COVID-19. So far, over 8.3 million patients worldwide have received LAGEVRIO.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
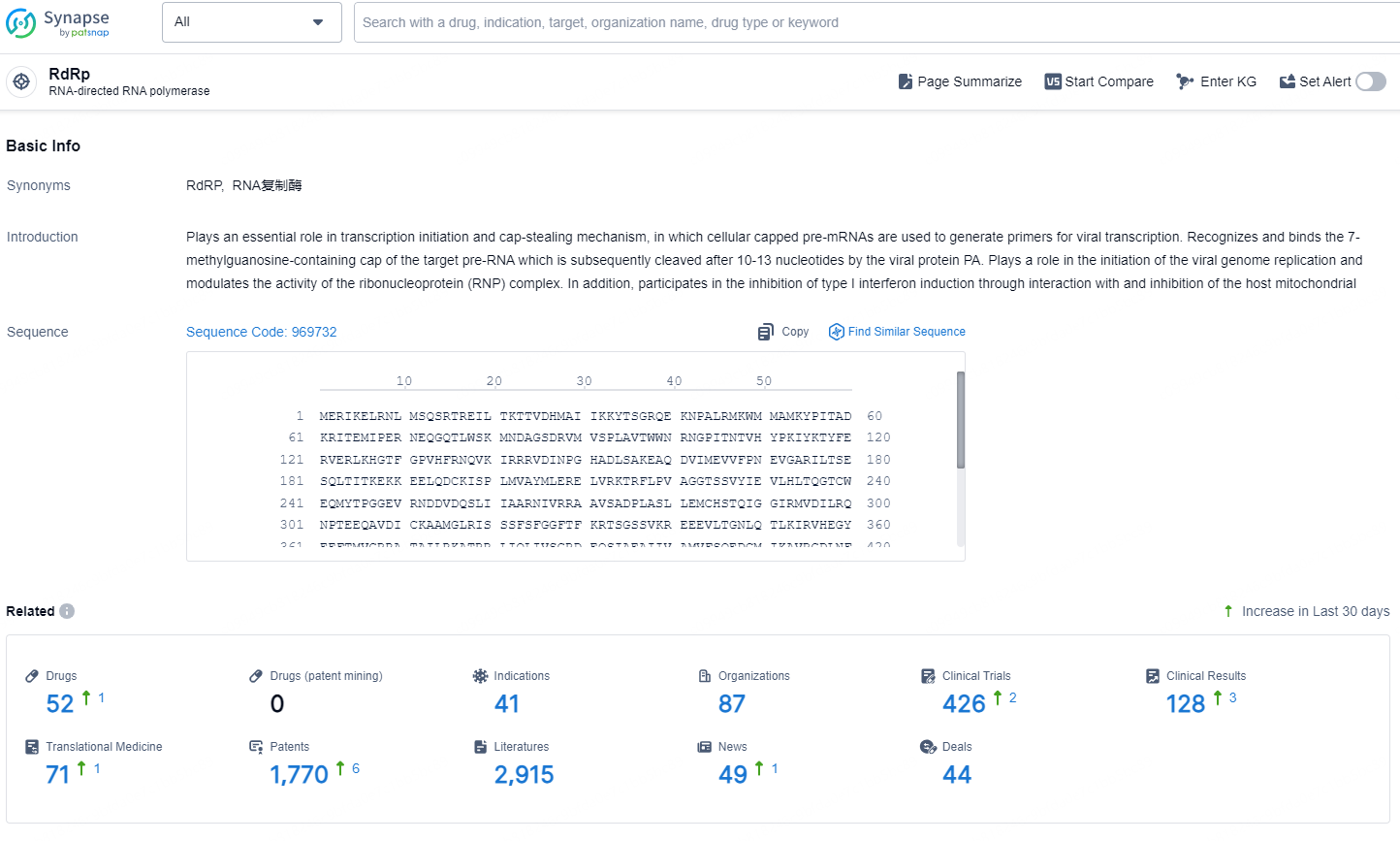
According to the data provided by the Synapse Database, As of December 10, 2024, there are 52 investigational drugs for the RdRp target, including 41 indications, 87 R&D institutions involved, with related clinical trials reaching 426, and as many as 1770 patents.
Molnupiravir is a small molecule drug that targets RdRp and is used in various therapeutic areas such as Infectious Diseases, Respiratory Diseases, Digestive System Disorders, Nervous System Diseases, and Other Diseases. The active indications for Molnupiravir include COVID-19, Respiratory Syncytial Virus Infections, Influenza, Human, Liver Diseases, Encephalomyelitis, and Venezuelan Equine.