Promising Phase 1 Results for Trishula Therapeutics' TTX-030 in Metastatic Pancreatic Cancer
Ryvu Therapeutics (WSE: RVU), a clinical-stage company dedicated to drug discovery and development, specializing in innovative small molecule therapies targeting new opportunities in oncology, announced the initiation of patient dosing in the REMARK study. This Phase II clinical trial is evaluating RVU120 as a standalone treatment for individuals with LR-MDS.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
“We are pleased to announce the launch of the REMARK study for RVU120, which holds the potential to aid patients with lower-risk MDS. This study builds on the encouraging outcomes observed in our Phase Ib trial involving patients with AML and high-risk MDS, where several patients showed hematologic improvement, including cases of transfusion independence. The aim is to further evaluate the safety and efficacy of RVU120 in patients with lower-risk MDS, supported by solid preclinical and mechanistic data. We are confident that this marks significant progress towards our goal of developing effective treatments for hematological diseases and providing new therapeutic options. I am thrilled to have the support of the EMSCO network and Prof. Uwe Platzbecker in this endeavor,” said Hendrik Nogai, M.D., Chief Medical Officer of Ryvu Therapeutics.
REMARK is an open-label, multicenter Phase II study investigating RVU120, a novel small-molecule cyclin-dependent kinase (CDK) 8/19 inhibitor. The study aims to address anemia in patients with lower-risk myelodysplastic syndromes (MDS). In REMARK, RVU120 is being tested as a single agent in patients with LR-MDS who have not responded to existing treatments.
The REMARK study is being conducted as an investigator-initiated study through the EMSCO network with Prof. Uwe Platzbecker, an internationally recognized expert in LR-MDS, as the Coordinating Principal Investigator.
"I am proud that we have initiated the REMARK study in alignment with our ambitious plans. RVU120 has demonstrated promising hematologic improvement in patients with compromised bone marrow function. I am optimistic that these clinical findings will translate to positive outcomes in the REMARK study. RVU120 presents important characteristics that should be considered as a potential new treatment option for patients with LR-MDS. It may help us achieve our ultimate goal of reducing the need for red blood cell transfusions in these patients," said Uwe Platzbecker, M.D., Director of the Clinic and Poliklinik for Hematology, Cell Therapy and Hemostaseology at the Leipzig University Hospital.
REMARK is being launched based on the clinical safety and efficacy data gathered so far, along with strong preclinical and mechanistic evidence. MDS pathogenesis is influenced by gene expression changes that impede the maturation of hematopoietic cells. RVU120 activates erythroid gene expression programs driven by STAT5 and GATA1 in abnormal stem cells from MDS patients. Importantly, RVU120’s activity does not cause significant toxicity in the hematopoietic system, positioning it as a promising candidate for treating transfusion-dependent MDS patients.
In REMARK, patients will receive RVU120 for at least 8 complete cycles (24 weeks). The primary objective is to achieve hematologic improvement in the form of an erythroid response (HI-E), with secondary objectives including independence from RBC transfusions, improvement in hemoglobin levels, quality of life, disease progression, and analysis of specific gene mutations.
REMARK represents the third of four planned RVU120 Phase II clinical studies scheduled for launch in 2024. Ryvu has already commenced patient treatment in the RIVER-52 and RIVER-81 studies in AML, and the fourth Phase II trial, the POTAMI-61 study, evaluating both monotherapy and combination therapy for treating patients with myelofibrosis (MF), is planned to start soon.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
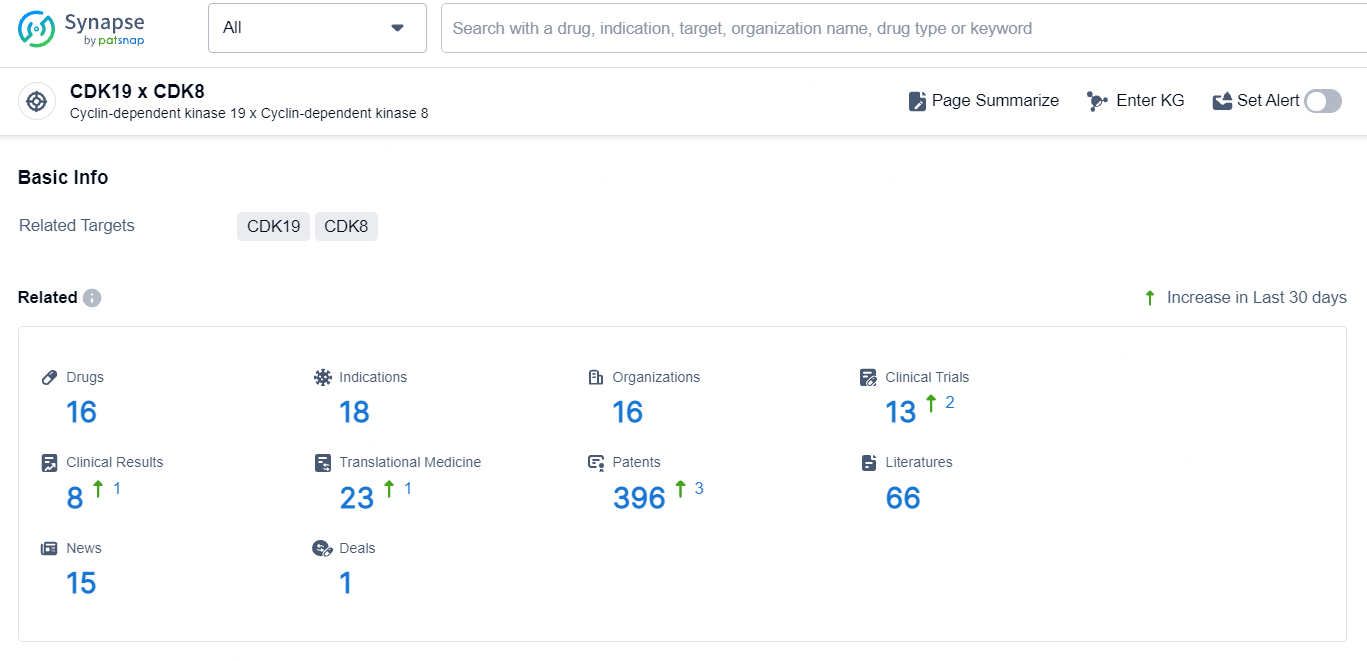
According to the data provided by the Synapse Database, As of September 23, 2024, there are 16 investigational drugs for the CDK19 x CDK8 targets, including 18 indications, 16 R&D institutions involved, with related clinical trials reaching 13, and as many as 396 patents.
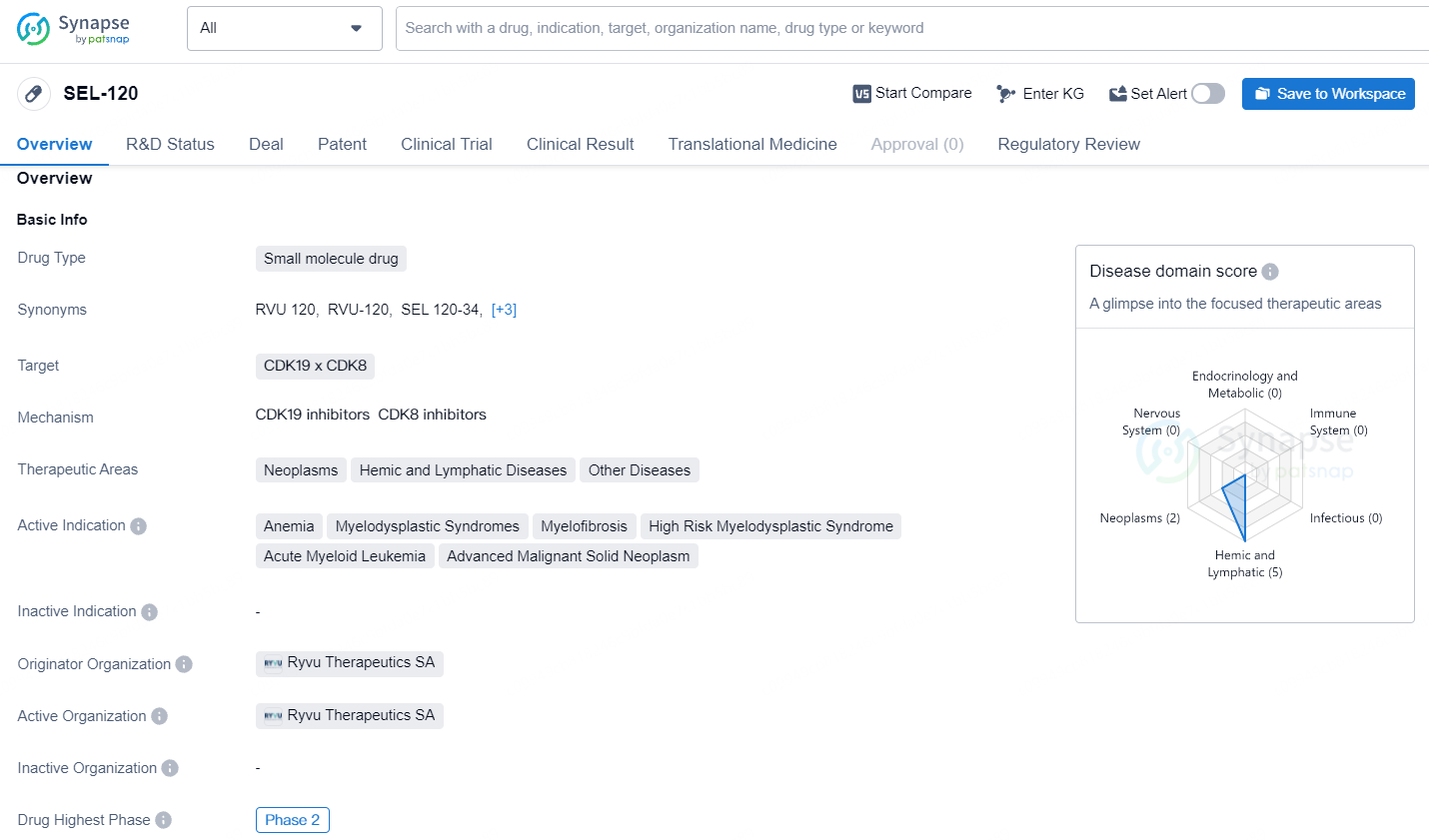
SEL-120 is a small molecule drug developed by Ryvu Therapeutics SA, with CDK19 x CDK8 as its targets. The drug falls within the therapeutic areas of Neoplasms, Hemic and Lymphatic Diseases, and Other Diseases. It is actively indicated for Anemia, Myelodysplastic Syndromes, Myelofibrosis, High Risk Myelodysplastic Syndrome, Acute Myeloid Leukemia, and Advanced Malignant Solid Neoplasm. The highest phase of development for SEL-120 is Phase 2, indicating that it has advanced beyond early-stage clinical trials.