Protara Announces Positive Phase 2 Results for TARA-002 in NMIBC Patients
Protara Therapeutics, Inc. (Nasdaq: TARA), a company in the clinical development phase focused on innovative therapies for cancer and rare diseases, has revealed findings from its ongoing Phase 2 open-label trial known as ADVANCED-2. This study is evaluating intravesical TARA-002, the firm's experimental cell-based treatment, in high-risk Non-Muscle Invasive Bladder Cancer (NMIBC) patients exhibiting carcinoma in situ or CIS (± Ta/T1) who are either unresponsive or naïve to Bacillus Calmette-Guérin (BCG) therapy. The complete response (CR) rate among patients with various BCG treatment history reached 72% (13 out of 18) at the six-month mark and 70% (14 out of 20) at any point in time, with a full 100% (9 out of 9) of patients sustaining a CR from three to six months. Moreover, two out of three patients preserved a CR at the nine-month evaluation. These findings will be presented today during a poster session at the 25th Annual Meeting of the Society of Urologic Oncology (SUO) in Dallas, Texas.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The study comprised 20 patients who could be assessed at the three-month mark, 18 patients evaluated at six months, and three patients at nine months, with data collection ending on November 19, 2024. Within the pivotal segment of the ADVANCED-2 trial involving BCG-Unresponsive individuals, the complete response (CR) rate was 100% (4/4) at the six-month point, and 80% (4/5) at any time. In the proof-of-concept group of BCG-Naïve patients, the CR rate was 64% (9/14) at six months and 67% (10/15) overall. TARA-002 exhibited a positive safety and tolerability profile, with no adverse events classified as Grade 2 or higher, and no patients ceased treatment because of side effects.
“These remarkable results for TARA-002 highlight significant effectiveness in a challenging-to-treat patient demographic,” stated Brian Mazzarella, MD, Vice President of Research at Urology America and an investigator in the ADVANCED-2 study. “The performance of TARA-002 across different BCG experience levels, along with its straightforward administration and minimal burden for clinicians, positions it as a promising treatment alternative for patients with NMIBC.”
“We are excited by these favorable six-month results, which confirm the potential of TARA-002 in treating NMIBC while providing an attractive product profile for both healthcare providers and patients,” commented Jesse Shefferman, CEO of Protara Therapeutics. “These positive findings, paired with our expansion of international sites, are expected to boost patient recruitment, and we anticipate releasing preliminary findings from the 12-month evaluable patient group in mid-2025.”
The majority of adverse reactions experienced were Grade 1 and temporary, with no occurrences of Grade 2 or higher treatment-related adverse events, according to the study investigators. None of the patients withdrew from the study due to adverse effects. The most frequently observed side effects aligned with standard reactions to bacterial immunopotentiation, like flu-like symptoms. The predominant urinary symptoms were associated with the effects of urinary tract instrumentation, such as bladder spasms, burning sensations, and urinary tract infections, most of which resolved shortly after administration or within a timeframe from a few hours to a few days.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
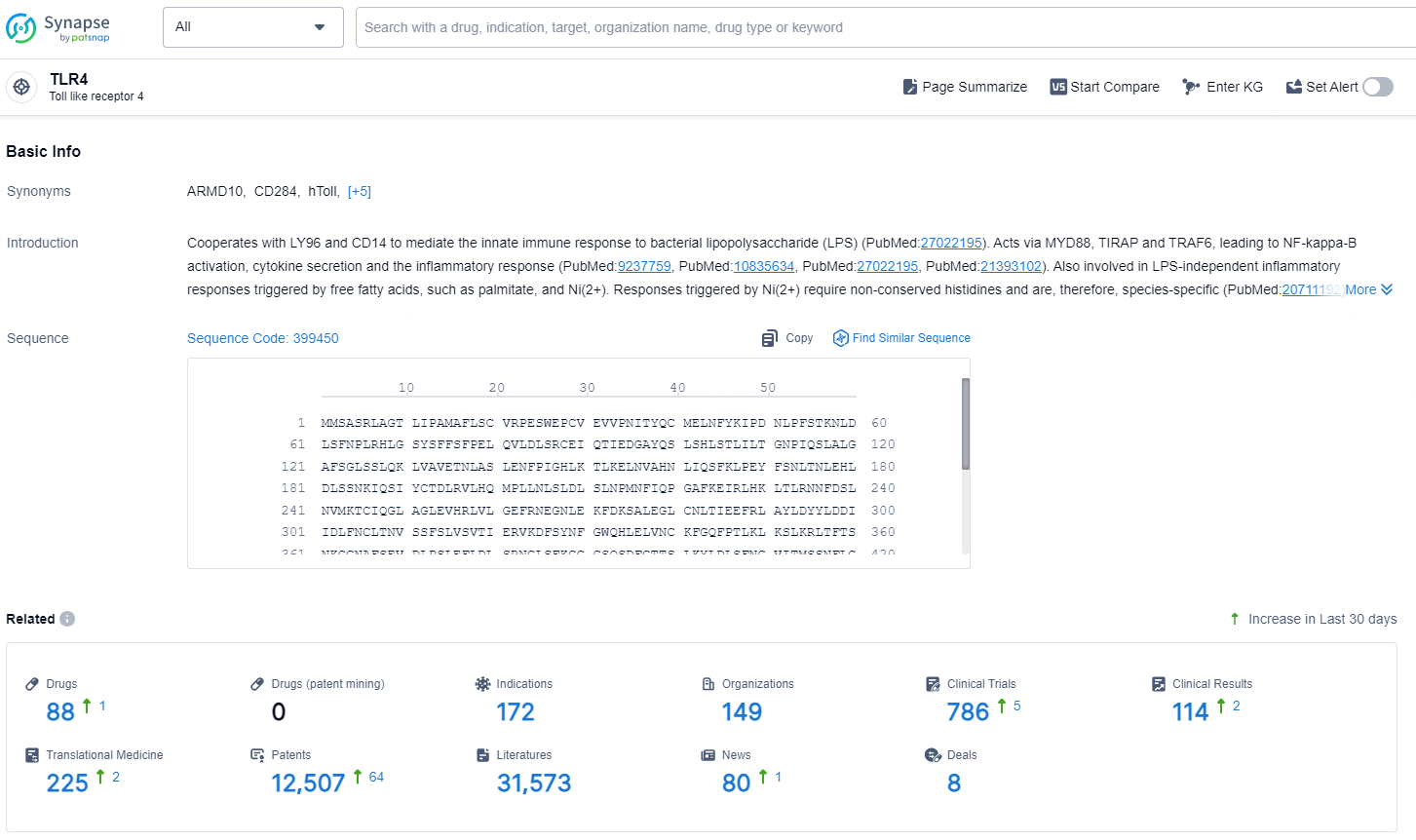
According to the data provided by the Synapse Database, As of December 17, 2024, there are 88 investigational drugs for the TLR4 target, including 172 indications, 149 R&D institutions involved, with related clinical trials reaching 786, and as many as 12507 patents.
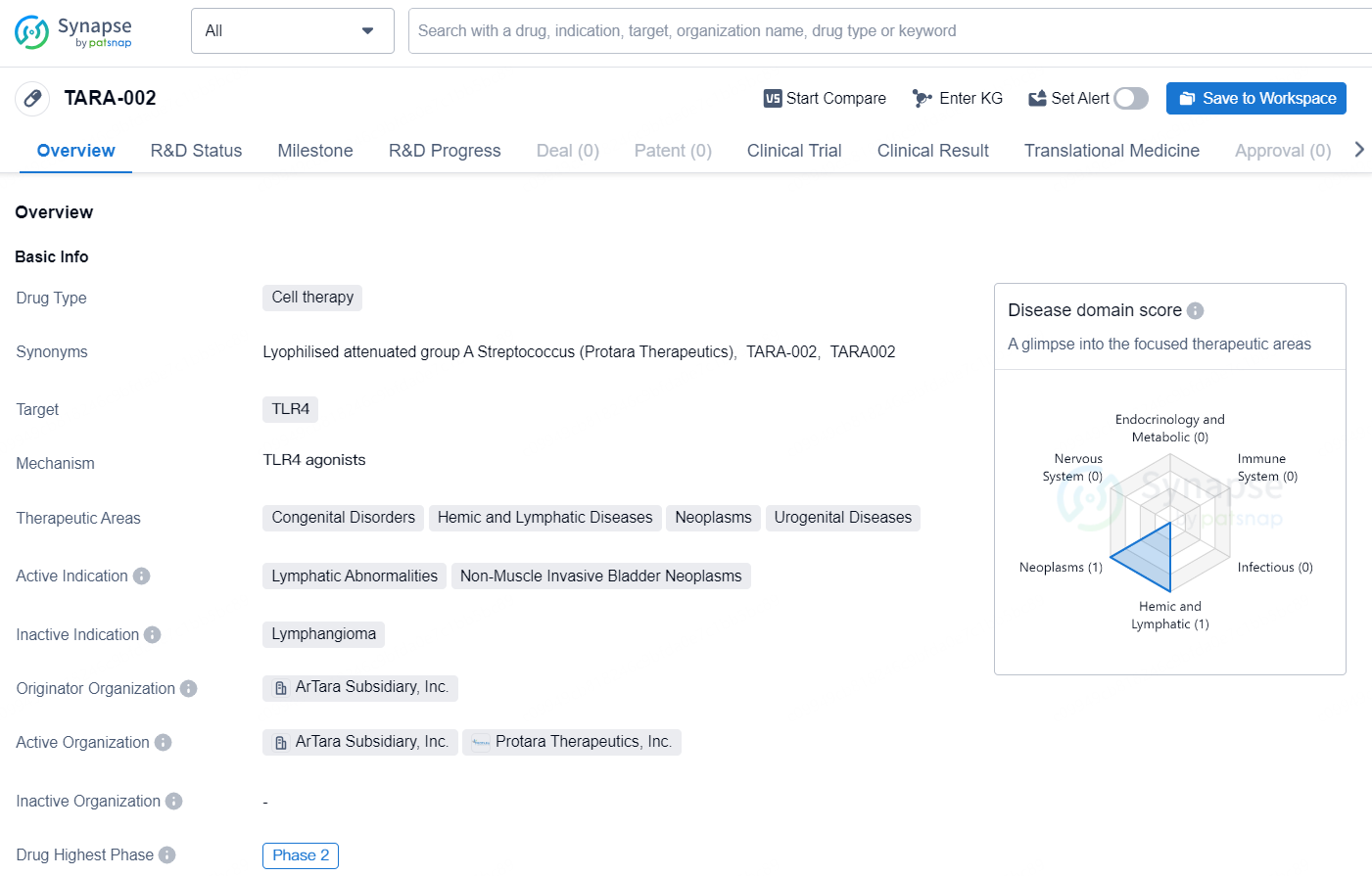
The drug TARA-002 is a cell therapy that targets TLR4, designed to treat a range of therapeutic areas including congenital disorders, hemic and lymphatic diseases, neoplasms, and urogenital diseases. Its active indication includes lymphatic abnormalities and non-muscle invasive bladder neoplasms. The drug is in its highest global development phase, Phase 2, and is being developed by the Originator Organization, ArTara Subsidiary, Inc.