PureTech Presents LYT-200 Data for AML/MDS at 2024 ASH Meeting
PureTech Health plc is a biotherapeutics firm in the clinical stage focused on transforming the lives of patients suffering from severe illnesses. The company showcased findings from the dose escalation segment of its ongoing Phase 1b trial, which investigates LYT-200, an innovative anti-galectin-9 monoclonal antibody, in individuals with relapsed or refractory acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS) at the 2024 Annual Meeting of the American Society of Hematology (ASH) held in San Diego, California.
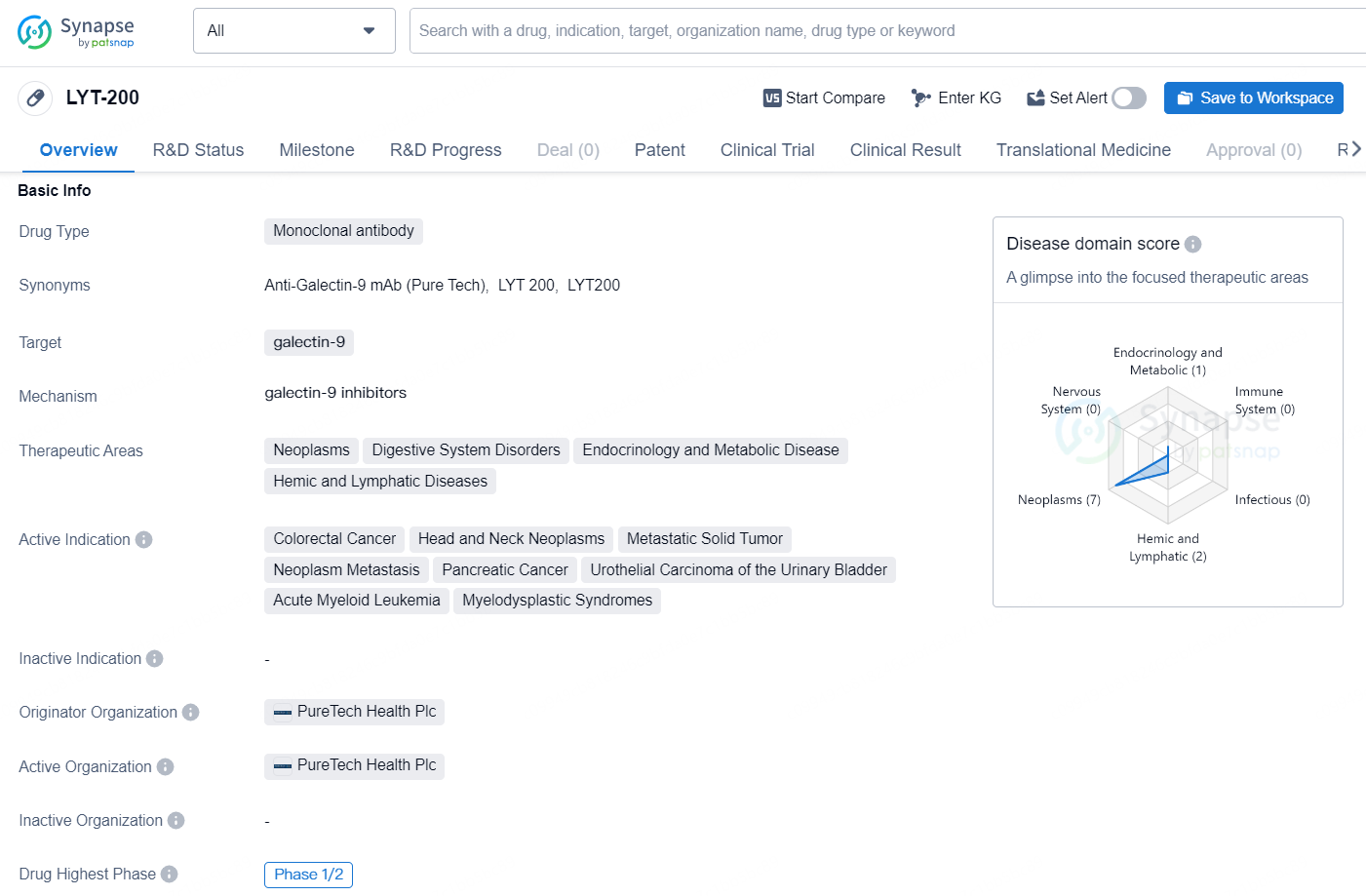
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
LYT-200 is under investigation as both a standalone treatment and in conjunction with the standard therapies venetoclax and hypomethylating agents (HMA) for patients whose disease has persisted or recurred after at least one previous line of treatment. The agent targets galectin-9, a glycan-binding protein that is notably elevated in acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS) and is pivotal in the processes of disease onset, progression, immune evasion, and resistance to therapies. Preliminary findings indicate a positive safety profile across both treatment groups and various dosages, with no dose-limiting toxicities reported, alongside signs of therapeutic response, improvements in hematological parameters, and extended disease management.
“Relapsed/refractory acute myeloid leukemia represents one of the most challenging cancer diagnoses, as 50% of patients do not respond to initial therapies or experience a relapse, leading to a median survival of under six months,” remarked Luba Greenwood, J.D., Entrepreneur-in-Residence at PureTech and lead on the Gallop Oncology initiative. “We are pleased to observe that LYT-200 has delivered responses and sustained disease control in patients with a history of extensive treatment, and we anticipate advancing LYT-200 as a crucial therapeutic alternative for the majority of AML cases.”
In the monotherapy cohort, LYT-200 was administered at five different dosage levels (ranging from 2.0 mg/kg to 16.0 mg/kg). The results showed that LYT-200 provided clinical benefit and prompted responses in heavily pre-treated individuals suffering from relapsed/refractory AML/MDS, including those with intricate cytogenetic profiles and mutations like KRAS, NRAS, and BRAF, as well as patients who had previously shown complete resistance to standard treatments. Out of 22 patients analyzed who participated in the monotherapy, 59% attained stable disease or an improved condition, with two achieving partial responses. The mean duration of treatment exceeded two months, surpassing the standard overall survival of about 1.7 months in individuals resistant to venetoclax/HMA.
When LYT-200 was used in combination with venetoclax/HMA, the findings suggest that it may augment the effectiveness of these standard therapies, even for those with relapsed or refractory conditions. Within the combination group, patients were given LYT-200 at three dosage levels (from 4.0 mg/kg to 12.0 mg/kg) in conjunction with venetoclax/HMA. Among 15 evaluable patients treated with the combination, 80% achieved stable disease or better, with two patients reaching complete responses and one attaining a morphologic leukemia-free state (MLFS). This combination approach has also shown clinical benefits for individuals with KRAS/NRAS mutations, with a mean treatment duration exceeding two months up to the data cutoff point.
“Galectin-9 is a critical factor in both the growth and immune evasion associated with AML, and it remains an untapped therapeutic target,” stated Aleksandra Filipovic, M.D., Ph.D., Head of Oncology at PureTech. “LYT-200 introduces an innovative strategy for addressing AML, leveraging a dual mechanism that induces cancer cell death through apoptosis and DNA damage while reactivating the immune system's primary anti-cancer responses. We are encouraged by these Phase 1 results that showcase the significant potential of this dual action of LYT-200, whether used alone or alongside current standard treatments.”
Pharmacodynamic evaluations of treated subjects, utilizing gene and protein assessments of patient cells, support the dual action mechanism of LYT-200 and uncover AML-related cellular pathways along with specific immune cell types that may be crucial for eliciting a response.
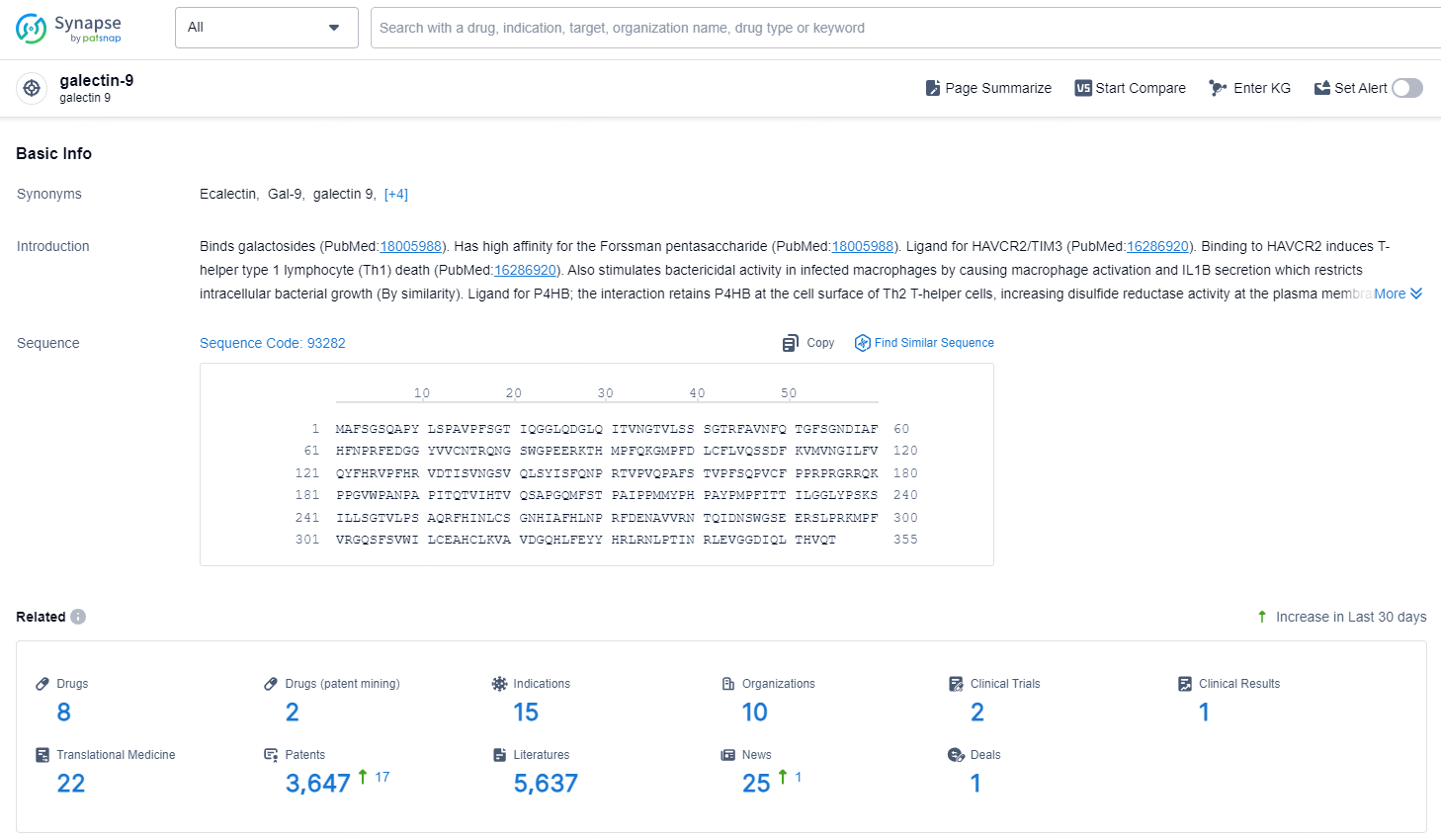
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Chemical, As of December 11, 2024, there are 8 investigational drugs for the galectin-9 target, including 15 indications, 10 R&D institutions involved, with related clinical trials reaching 2, and as many as 3647 patents.
LYT-200 is a monoclonal antibody that targets galectin-9 and is being developed for the treatment of various therapeutic areas including neoplasms, digestive system disorders, endocrinology and metabolic disease, and hemic and lymphatic diseases.