Rallybio Unveils Phase 1 Data for Single Ascending Dose of RLYB116, a Novel Subcutaneous Complement Component 5 Inhibitor
Rallybio Corporation, a clinical-stage biotech firm focused on discovering and expediting the production of ground-breaking therapies for patients battling severe and seldom diseases, unveiled clinical data today via a poster from the first human Phase 1 clinical trial's single ascending dose study on healthy subjects of RLYB116.
RLYB116, a potential long-acting solution, is a subcutaneously administered C5 inhibitor which is currently being developed for managing patients with complement-mediated diseases. The poster was showcased at the 29th International Complement Workshop held in Newcastle, UK.
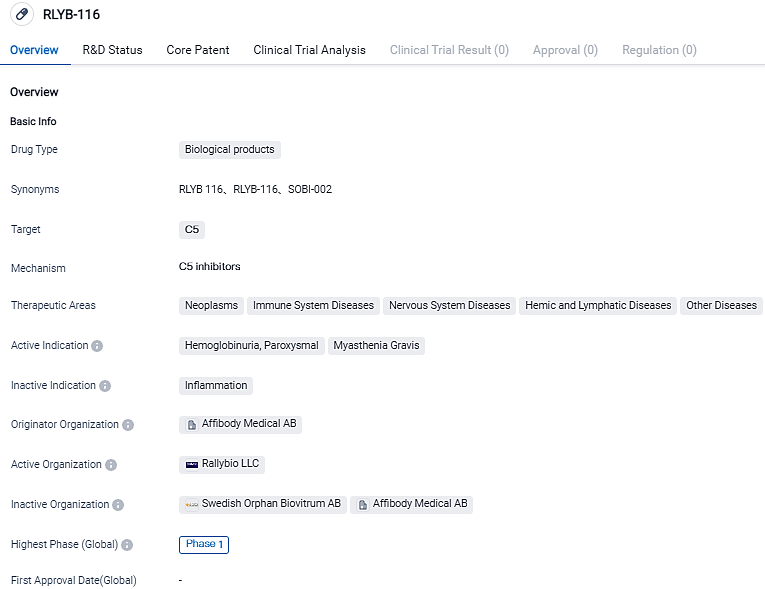
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The evidence showed that the single-dose application of RLYB116 at two higher doses, 100 mg and 300 mg, resulted in maximum exposures exceeding 1 µM and 3 µM, respectively, and more than 99% reductions in free C5 concentrations. RLYB116, when applied subcutaneously, was generally seen to be well-tolerated for a singular 100 mg or 300 mg dosage, with minor to moderate side effects and no severe drug-related adverse reactions.
Eric Watsky, M.D., the Rallybio RLYB116 Program's Leader, remarked, "The data debuted today at ICW consistently backs our conviction in RLYB116's prospective utilization for treating a variety of complement-mediated diseases.
The data from this singular dose implies RLYB116 might be a highly inventive C5 inhibitor with the capacity to address substantial unfulfilled medical necessities for patients". "We remain on course to reveal preliminary results from the Phase 1 multiple ascending dose study of RLYB116 and announce our indication strategy in the last quarter of 2023."
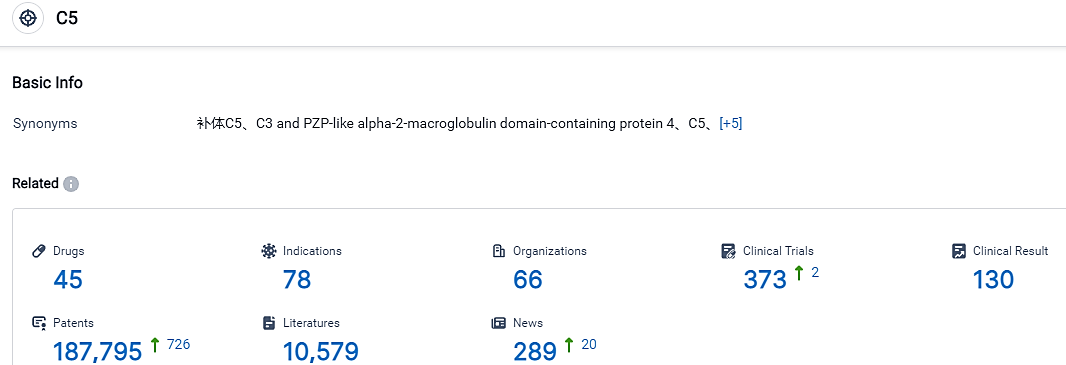
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of September 5, 2023, there are 45 investigational drugs for the C5 target, including 78 applicable indications, 66 R&D institutions involved, with related clinical trials reaching 373, and as many as 187795 patents. Bear in mind that the details shared are impartial, only relying on the presented figures. To establish the efficiency and security of RLYB-116 in addressing specified ailments, supplementary examinations and clinical tests are compulsory.