Sacituzumab tirumotecan: A Quick Look at Its R&D Progress and Clinical Results from the 2024 AACR
TROP2 (trophoblast cell surface antigen 2) is commonly over-expressed in non-small cell lung cancer (NSCLC) and associated with poor prognosis. SKB264 (MK-2870) is a TROP2 ADC developed with a novel linker to conjugate the payload, a belotecan-derivative topoisomerase I inhibitor. The linker is affected by both extracellular pH-sensitive cleavage and intracellular enzymatic cleavage within tumor cells, which leads to efficiently release payload inside tumor cells as well as within tumor microenvironment to exert its anti-tumor effects. On April 5, 2024, the 2024 AACR present the updated data from a Phase 2 expansion cohort for patients (pts) with advanced NSCLC.
Sacituzumab tirumotecan's R&D Progress
Sacituzumab tirumotecan (MK-2870) is an antibody drug conjugate (ADC) that targets Trop-2, a protein found in various types of cancers. It has shown potential therapeutic benefits in treating a wide range of neoplasms, skin and musculoskeletal diseases, digestive system disorders, respiratory diseases, urogenital diseases, other diseases, and endocrinology and metabolic diseases.
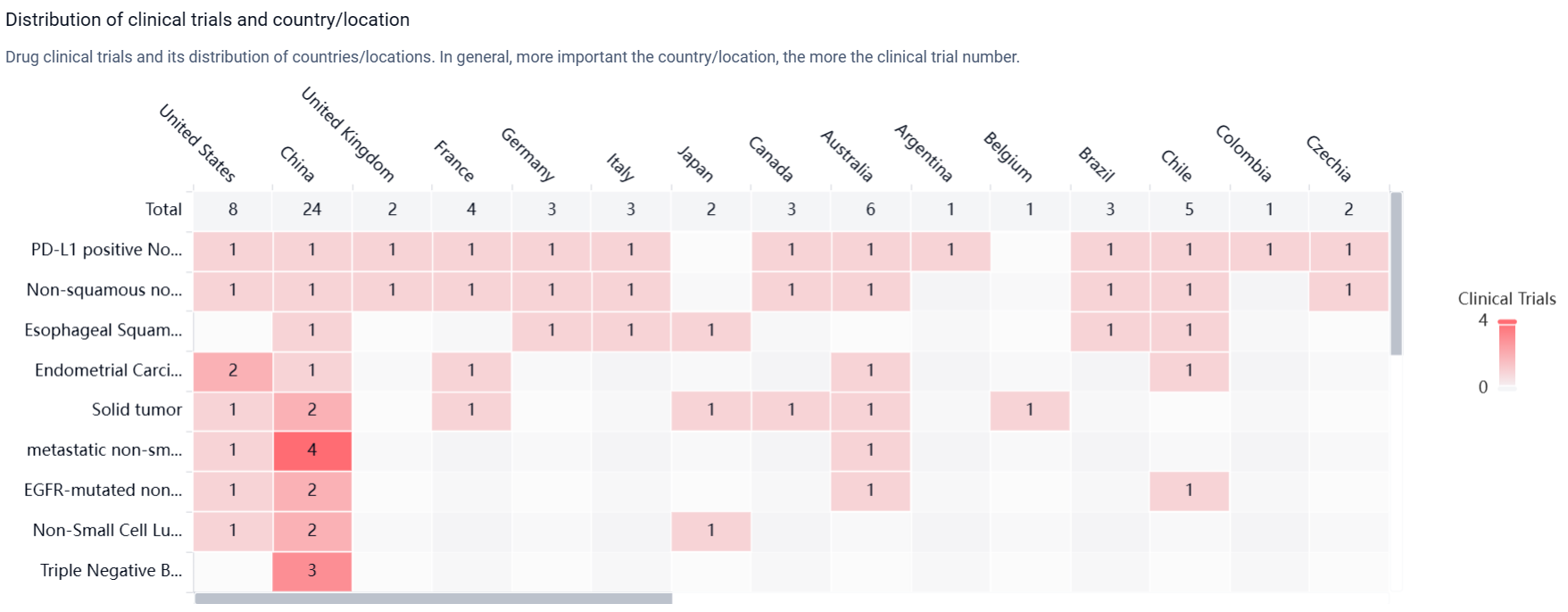
According to the Patsnap Synapse, Sacituzumab tirumotecan was developed by Sichuan Kelun Pharmaceutical Co., Ltd., a reputable organization in the pharmaceutical industry. It has reached the highest phase of development, which is the New Drug Application (NDA) or Biologics License Application (BLA) phase, both globally and in China. And the clinical trial distributions for Sacituzumab tirumotecan are primarily in the United States, China and United Kingdom. The key indication is PD-L1 positive Non-Small Cell Lung Cancer.
Detailed Clinical Result of Sacituzumab tirumotecan
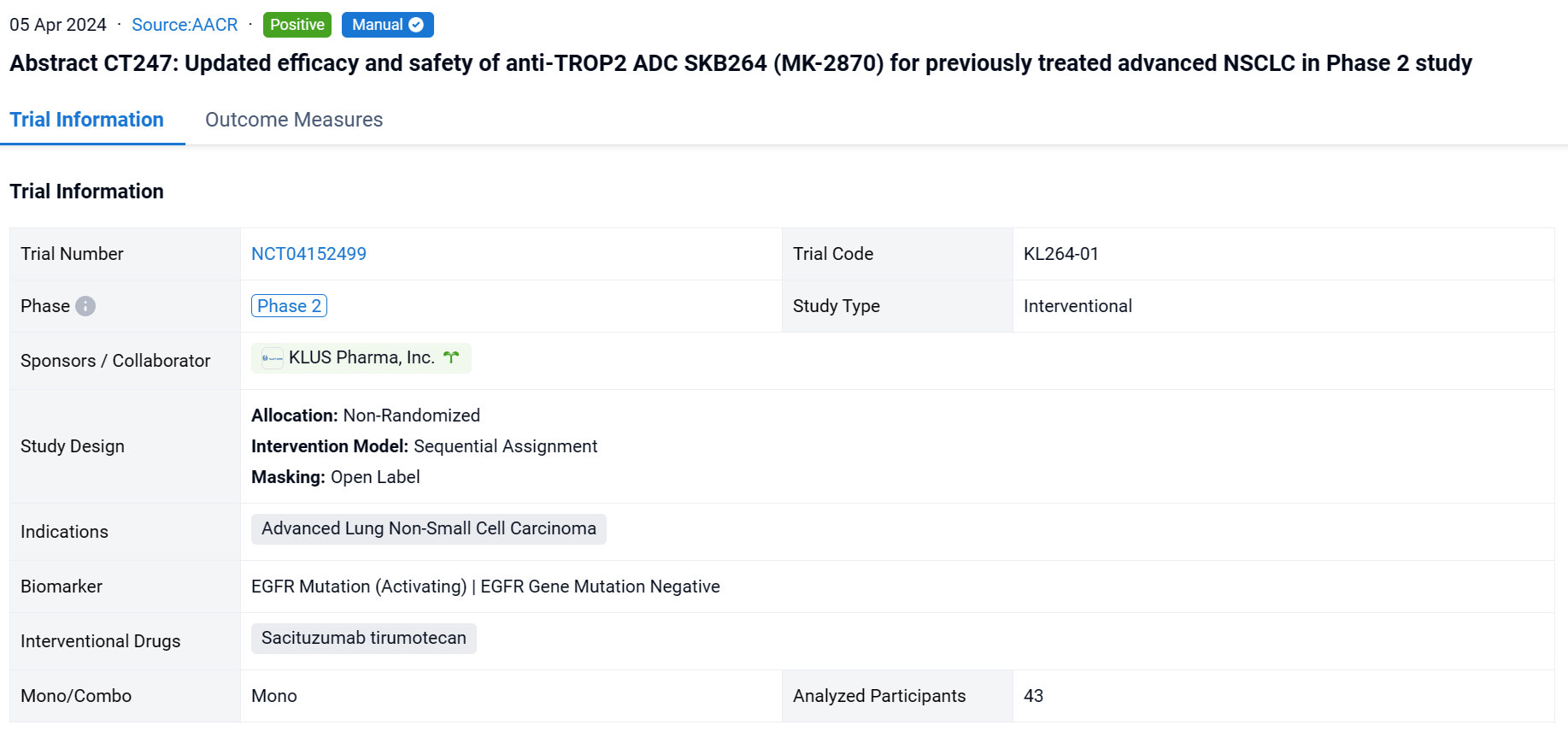
This non-randomized, sequential assignment, open-labeled clinical study (NCT04152499) was aimed to efficacy and safety of anti-TROP2 ADC SKB264 (MK-2870) for previously treated advanced NSCLC.
In this study, pts with previously treated advanced NSCLC were enrolled to receive SKB264 at 5 mg/kg Q2W until disease progression or unacceptable toxicity (KL264-01, NCT04152499). Tumor assessment was performed every 8 weeks per RECIST v1.1 assessed by investigator.
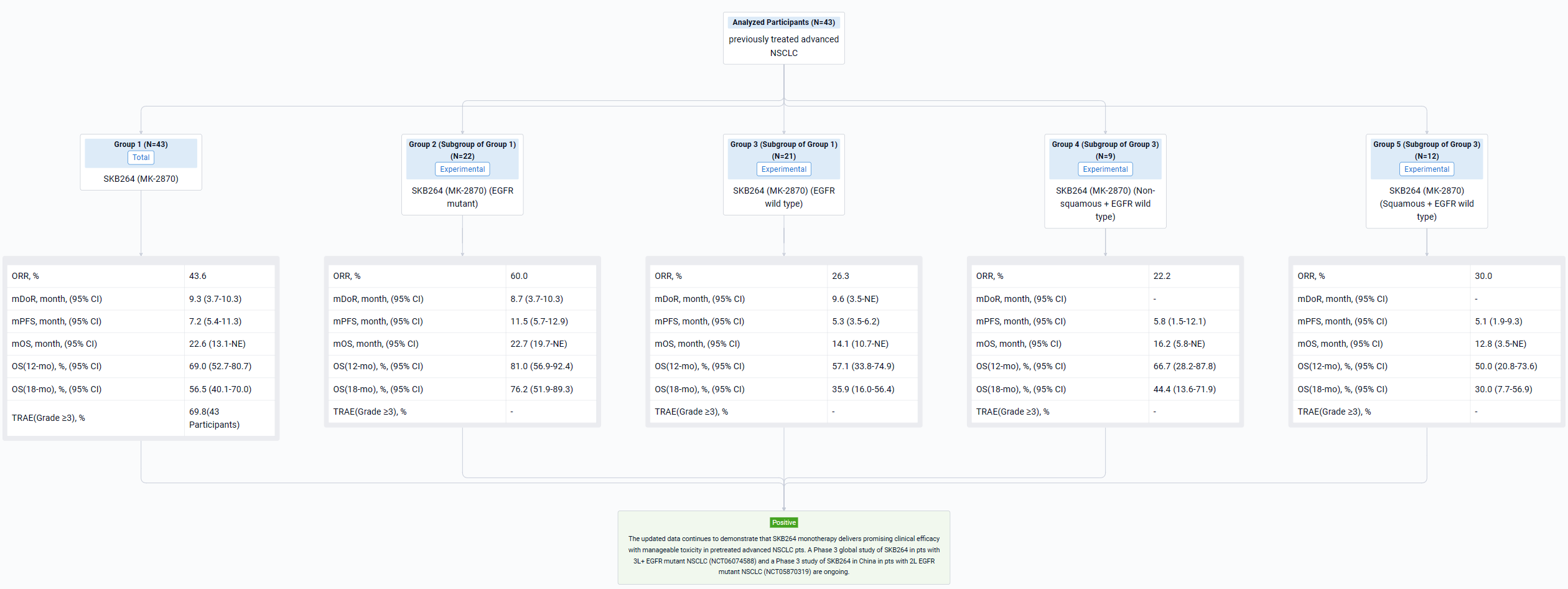
The result showed that as of Nov 22, 2023, 43 NSCLC pts had been enrolled. Median follow-up was 17.2 months (mo). 21 pts with EGFR wild type had received median prior 3 regimens of therapy including anti-PD-1/L1 inhibitors. 22 pts with EGFR mutant had progressed on or after TKI therapy, 50% of whom also failed at least one line of chemotherapy. Updated efficacy results are shown in the Table. Overall, 30 pts (69.8%) experienced Grade ≥3 treatment-related adverse events (TRAEs). The most common Grade ≥3 TRAEs were neutrophil count decreased (34.9%), anemia (30.2%), WBC count decreased (25.6%), stomatitis (9.3%), and rash (7.0%). No TRAEs leading to treatment discontinuation or deaths occurred. No drug-related ILD/pneumonitis was reported.
It can be concluded that the updated data continues to demonstrate that SKB264 monotherapy delivers promising clinical efficacy with manageable toxicity in pretreated advanced NSCLC pts. A Phase 3 global study of SKB264 in pts with 3L+ EGFR mutant NSCLC (NCT06074588) and a Phase 3 study of SKB264 in China in pts with 2L EGFR mutant NSCLC (NCT05870319) are ongoing.
How to Easily View the Clinical Results Using Synapse Database?
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
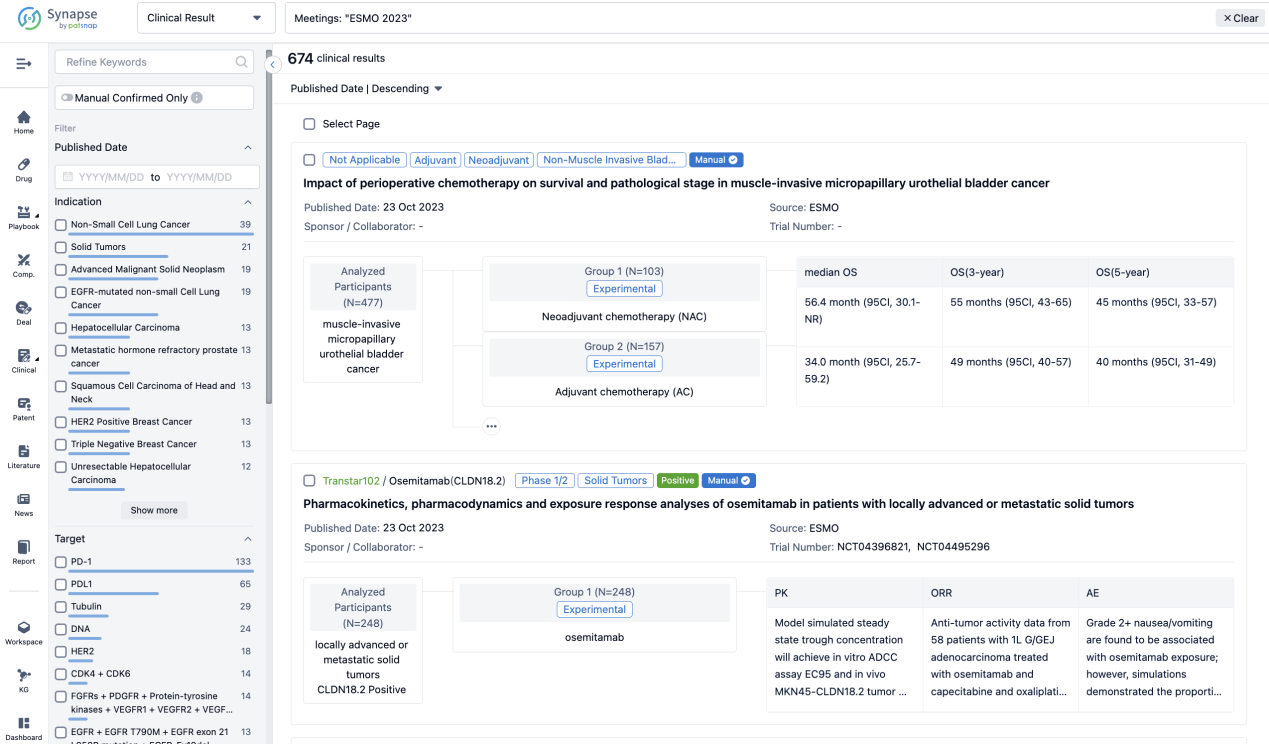
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.

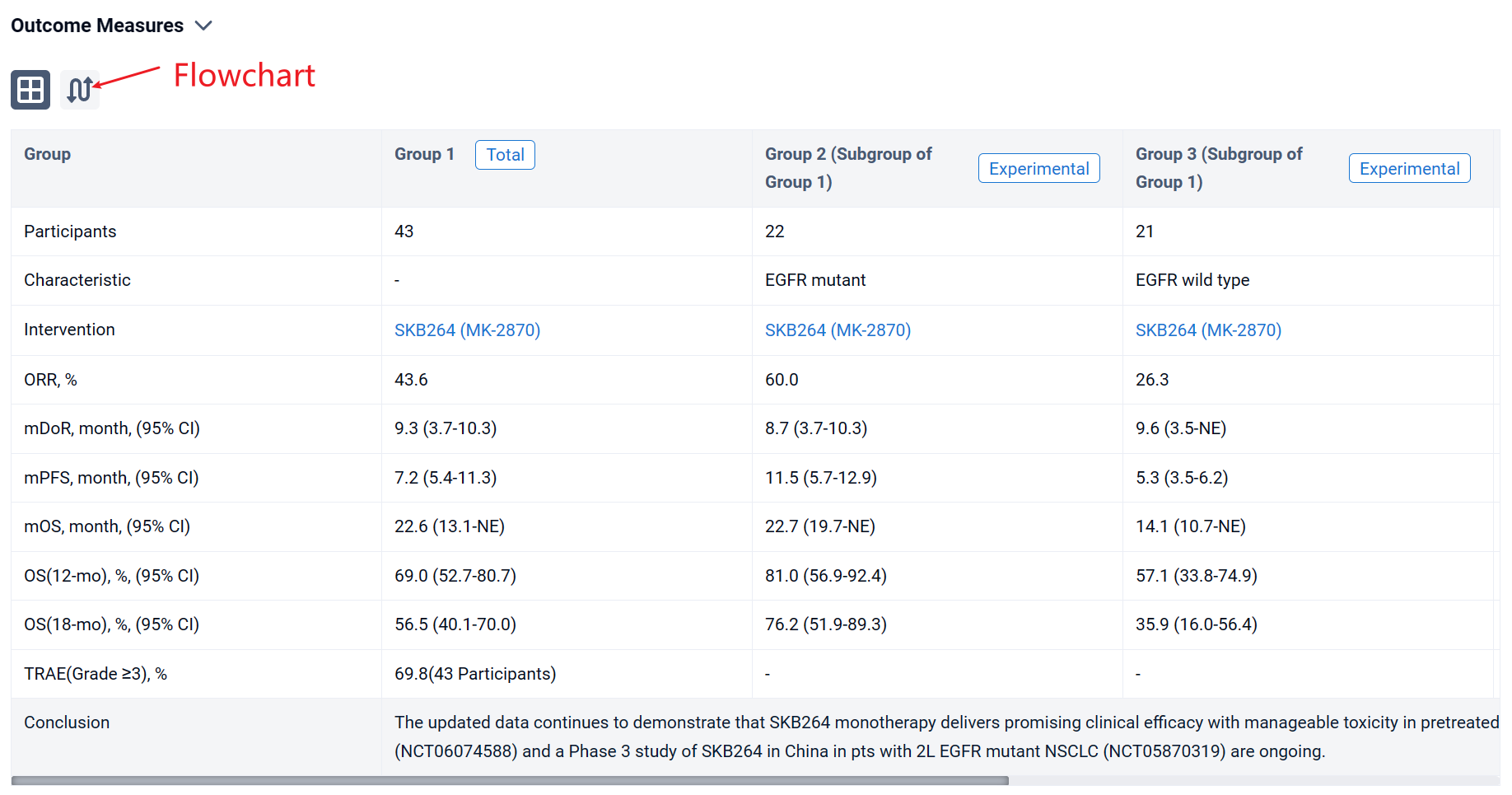
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.

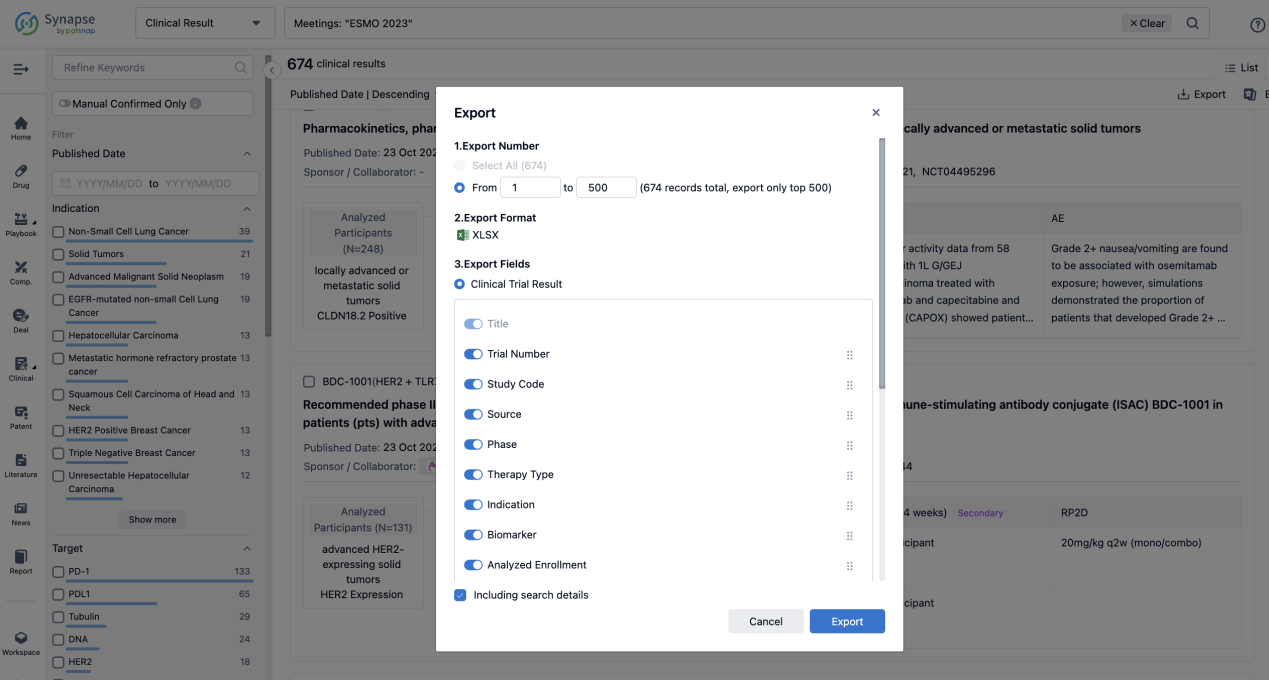
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!