Sanofi Joins Forces with RadioMedix and Orano Med to Pioneer Radioligand Therapies for Rare Cancers
On September 12th, Sanofi announced its entry into the nuclear medicine field through an exclusive licensing agreement with RadioMedix and Orano Med to jointly develop next-generation radioligand therapies (RLT) for rare cancers. According to the terms of the agreement, RadioMedix and Orano Med will receive a €100 million upfront payment and up to €220 million in sales milestone payments, along with eligibility for tiered royalties. This move marks Sanofi's official venture into the nuclear medicine sector, joining the ranks of global giants such as Novartis, Eli Lilly, and BMS.

Focus on Nuclear Medicine
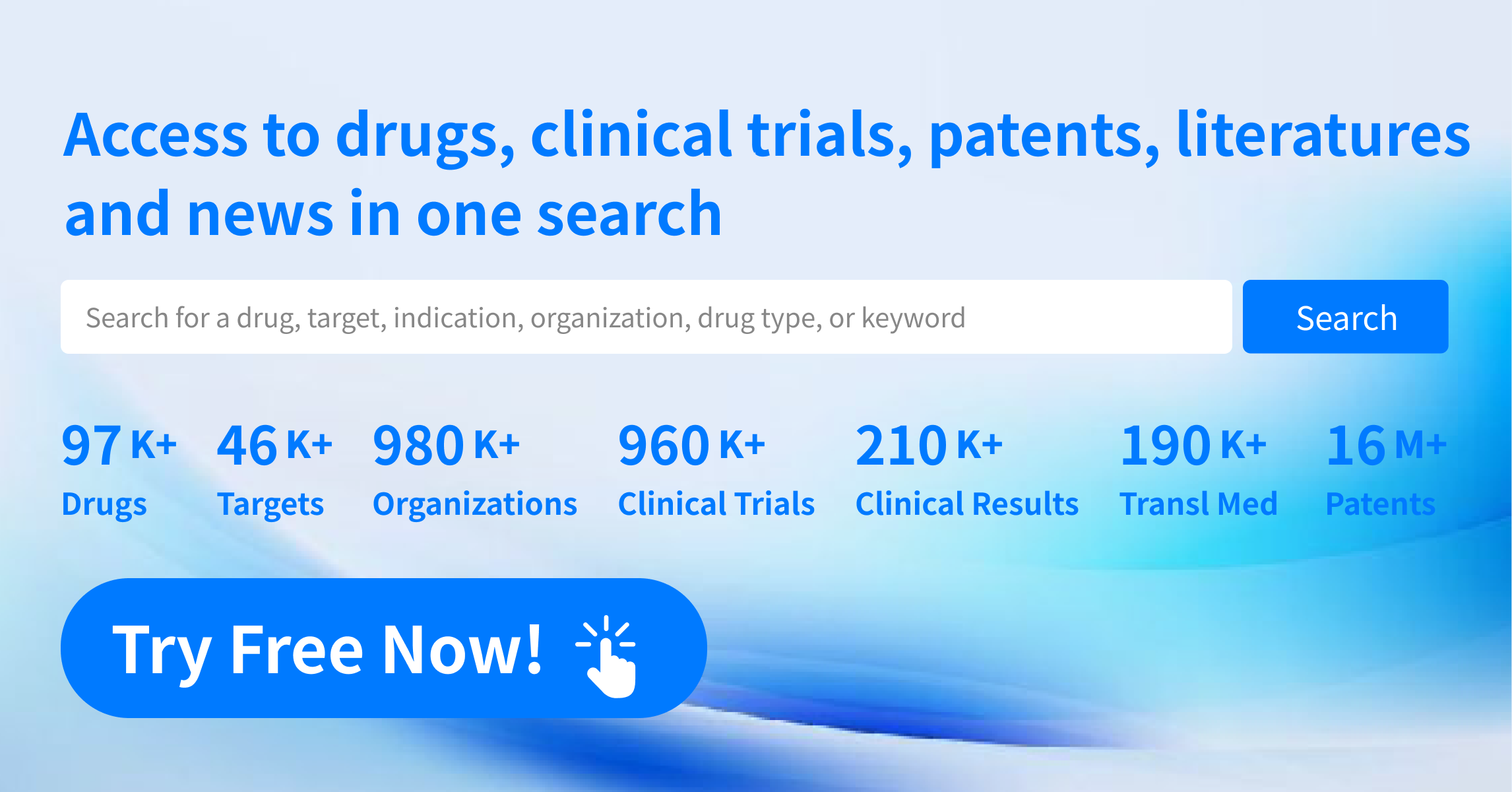
Below is an overview of the global clinical development pipeline for nuclear medicine.
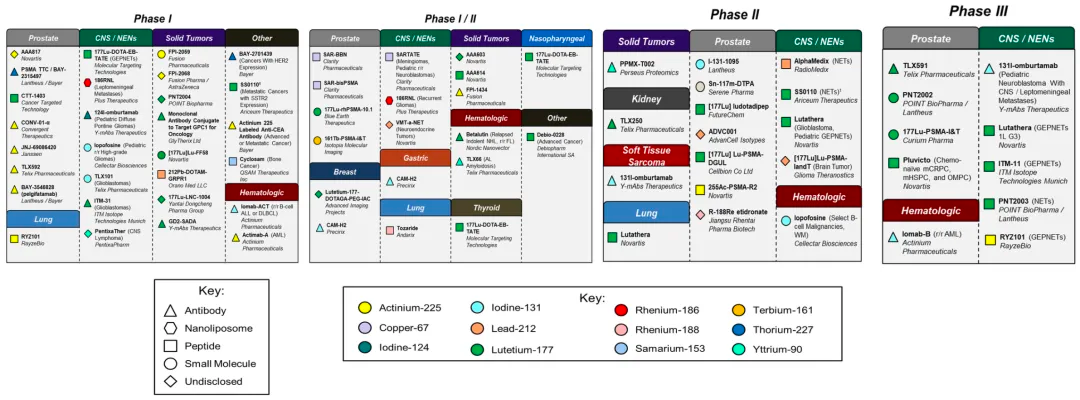
The radioligand therapy sector has attracted significant transaction activity in recent years, driven by promising market growth expectations. In 2023, venture capital transactions in this field increased by approximately 550%, surpassing $400 million. With more than 60 players already participating, this number is expected to rise as these therapies achieve greater clinical and commercial success.
About AlphaMedix™
RadioMedix is a US-based clinical-stage biotechnology company specializing in developing radiopharmaceuticals for PET imaging and targeted alpha therapy (TAT) to address unmet medical needs in cancer treatment. Orano Med, a French clinical-stage biotech company, focuses on developing lead-212 (212-Pb) radioligand therapies for cancer.
The core project of this collaboration is AlphaMedix™ (212Pb-DOTAMTATE), an original targeted alpha therapy by Orano Med. It consists of a peptide complex targeting somatostatin receptors and is labeled with lead-212 (212Pb) as an in vivo alpha particle generator. AlphaMedix™ is currently being evaluated for treating adult patients with inoperable or metastatic, progressive neuroendocrine tumors (NETs) that express somatostatin receptors.
The US FDA has granted AlphaMedix™ Breakthrough Therapy Designation for treating patients with gastroenteropancreatic neuroendocrine tumors (GEP-NETs) who have not undergone peptide receptor radionuclide therapy (PRRT). This designation is based on Phase I and II clinical trial results showing AlphaMedix's favorable tolerability and an overall response rate of 62.5%, assessed by RECIST 1.1 criteria.
Neuroendocrine tumors (NETs) are a heterogeneous group of rare cancers originating from neuroendocrine cells. These cancers primarily occur in the gastrointestinal tract and pancreas but can also arise in other tissues, including the thymus, lung, and less commonly in the ovaries, heart, and prostate. Most NETs strongly express somatostatin receptors. In the United States, approximately 12,000 patients are diagnosed with neuroendocrine tumors annually, with an average five-year survival rate of 60% at the metastatic stage. Despite a rising global prevalence of NETs each year, it is considered a rare cancer, affecting an estimated 35 per 100,000 people.
A key feature of nuclear medicine is its one-stop approach to diagnosis and treatment, as demonstrated by early 212-Pb development data.
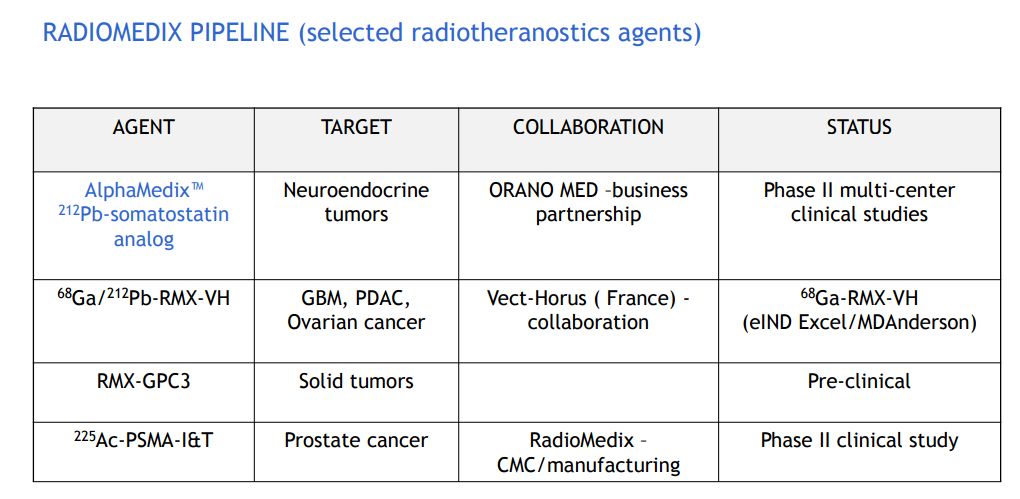
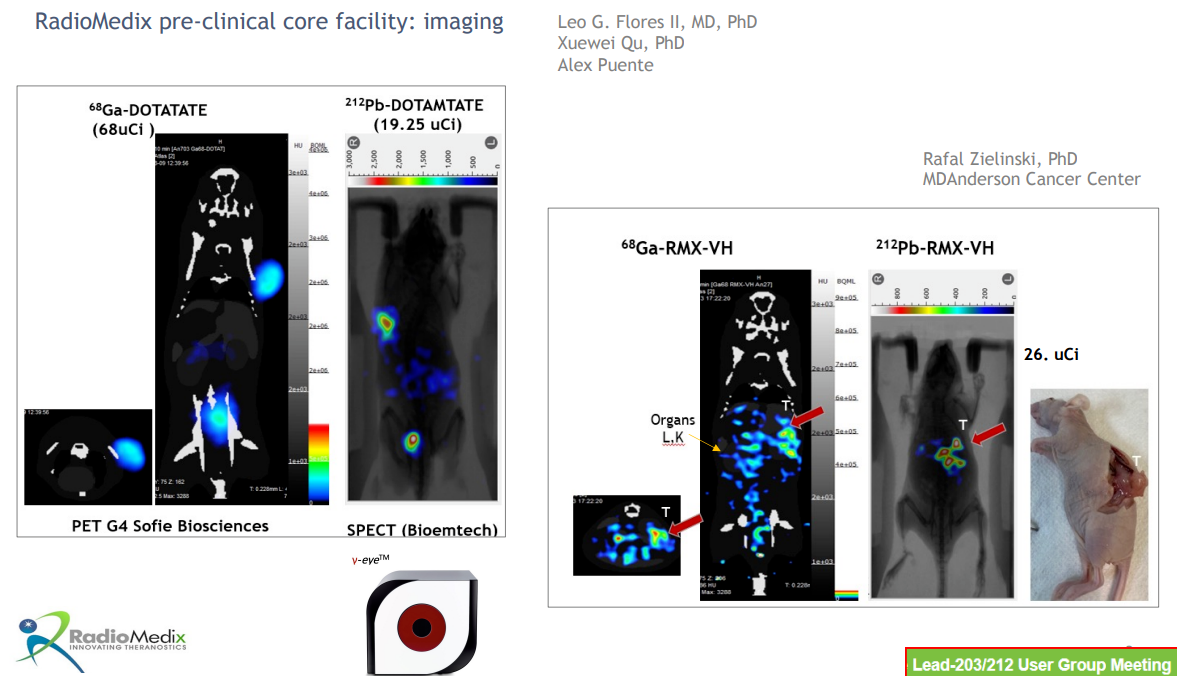
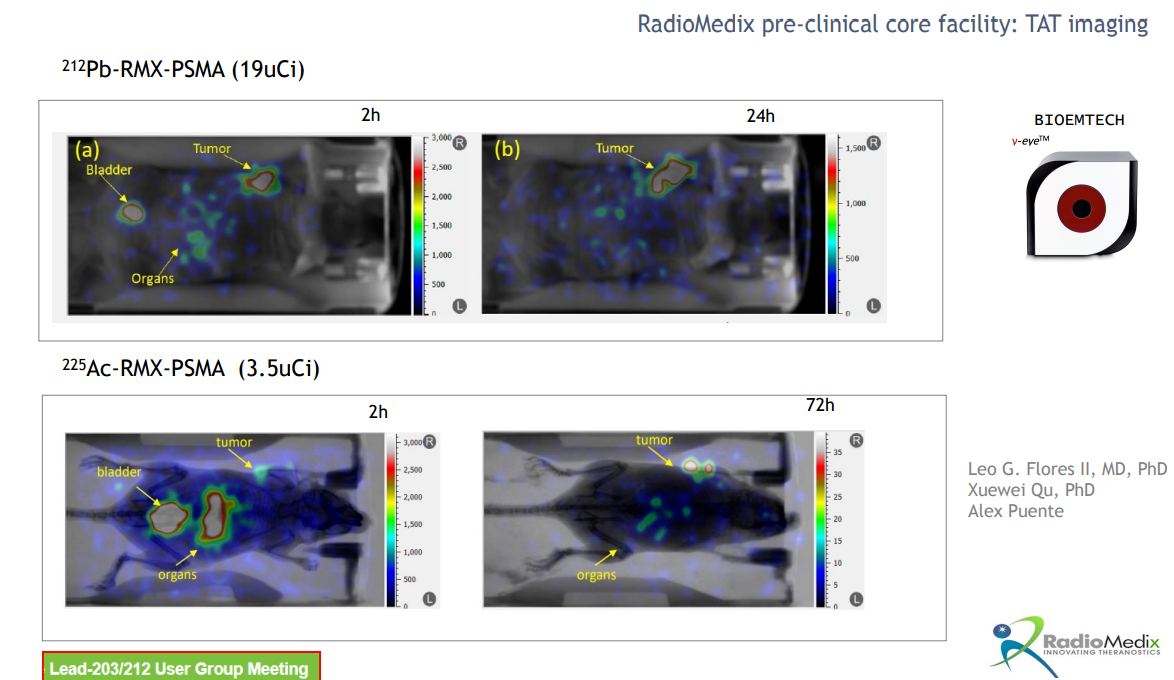
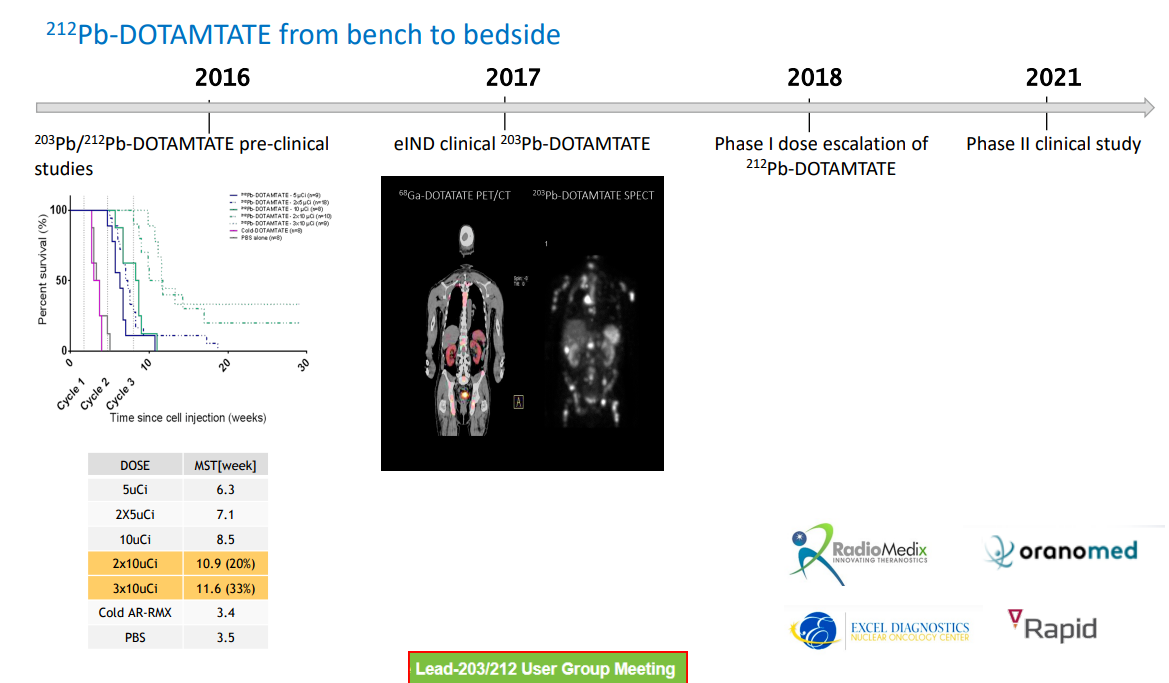
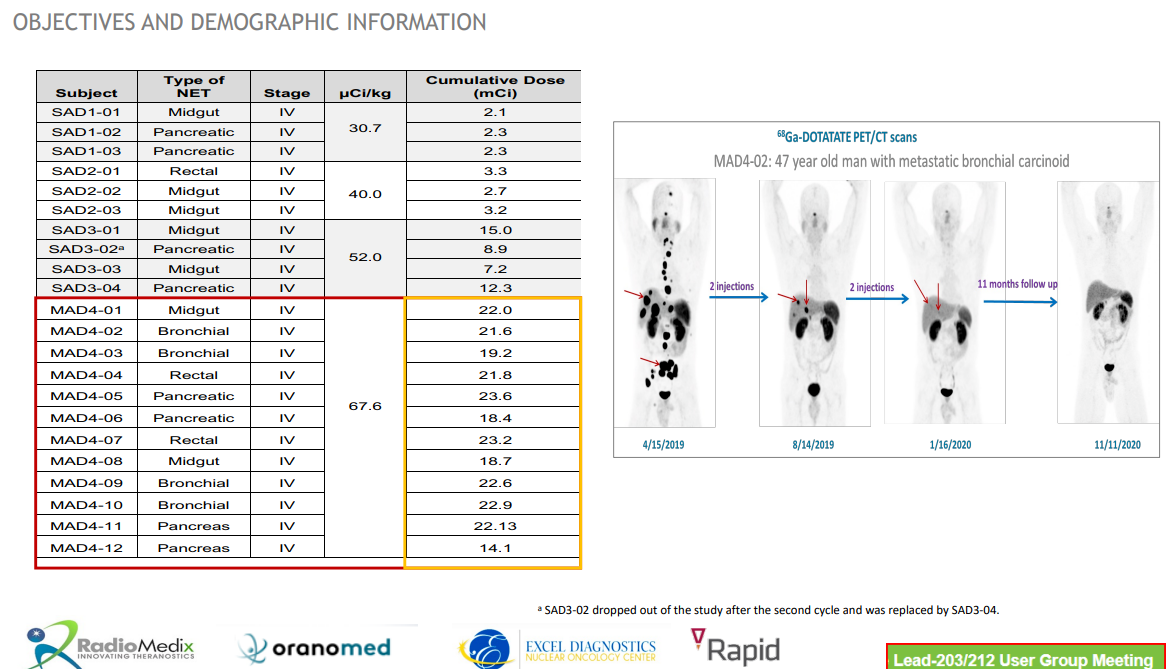 Dietmar Berger, Global Head of Development and Chief Medical Officer at Sanofi, said, "We are excited to develop pioneering projects in the rapidly evolving field of radioligand therapy for rare cancers. The early results of 212-Pb have already showcased its unique biophysical and clinical properties, reinforcing its potential as a transformative radioligand therapy for patients with challenging-to-treat rare cancers. This agreement underscores our commitment to exploring innovative technologies to address the needs of cancer patients."
Dietmar Berger, Global Head of Development and Chief Medical Officer at Sanofi, said, "We are excited to develop pioneering projects in the rapidly evolving field of radioligand therapy for rare cancers. The early results of 212-Pb have already showcased its unique biophysical and clinical properties, reinforcing its potential as a transformative radioligand therapy for patients with challenging-to-treat rare cancers. This agreement underscores our commitment to exploring innovative technologies to address the needs of cancer patients."
Understanding Sanofi
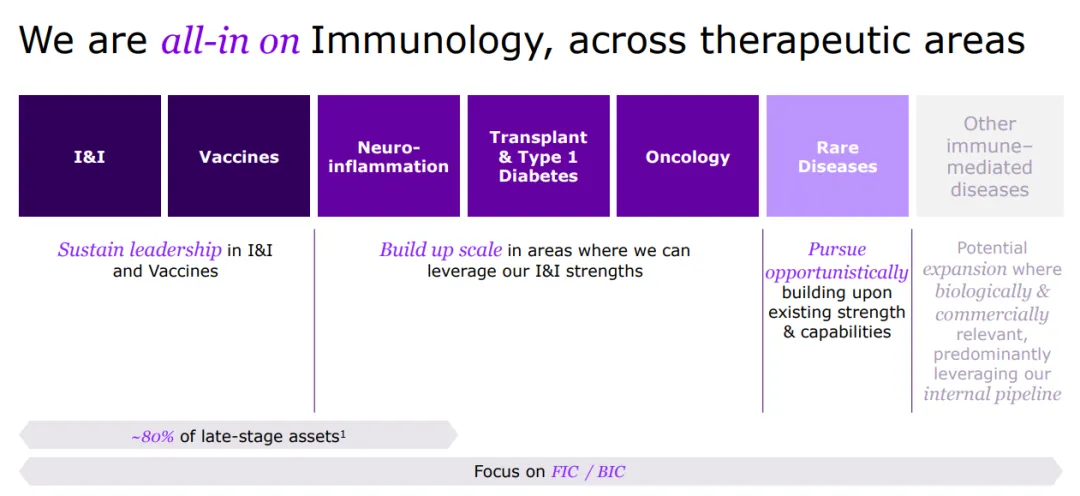
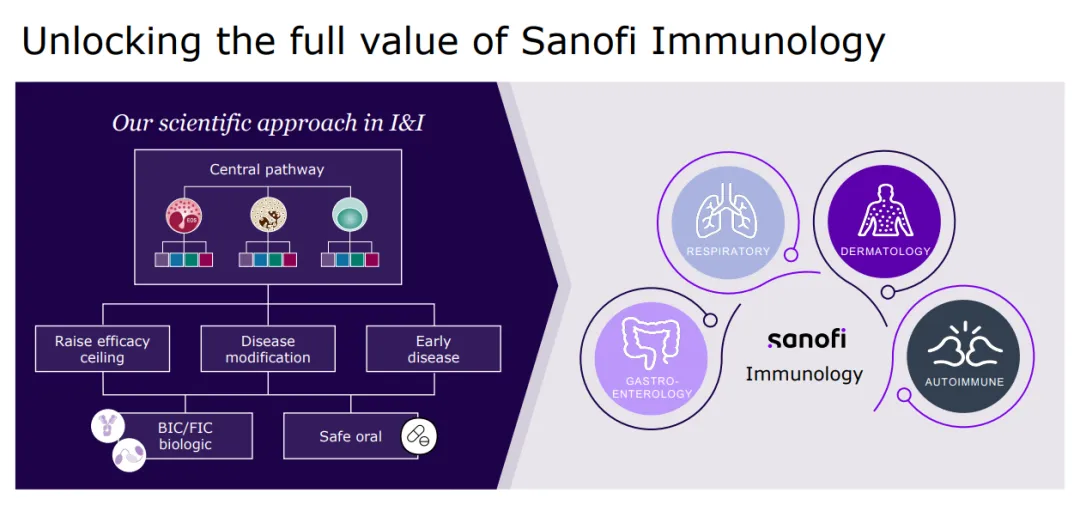
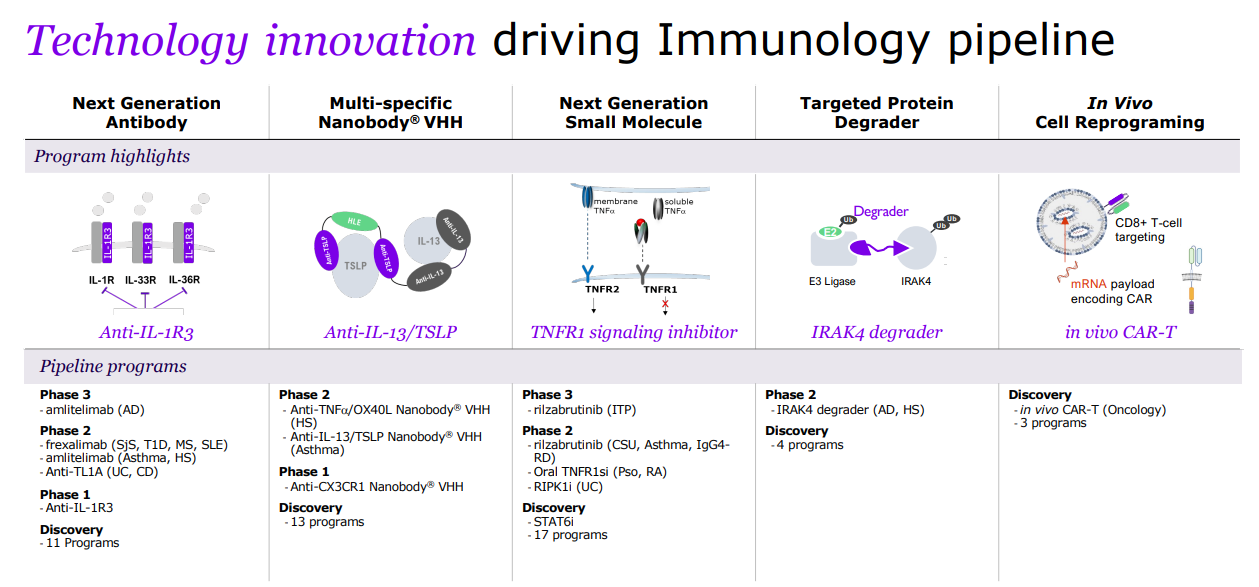
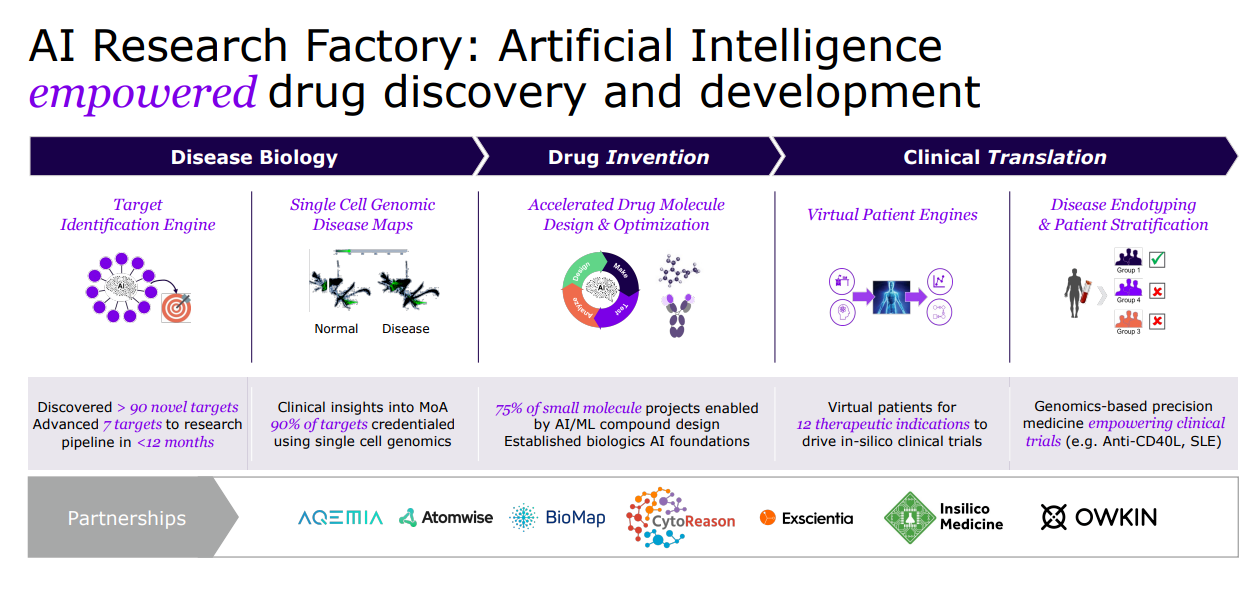
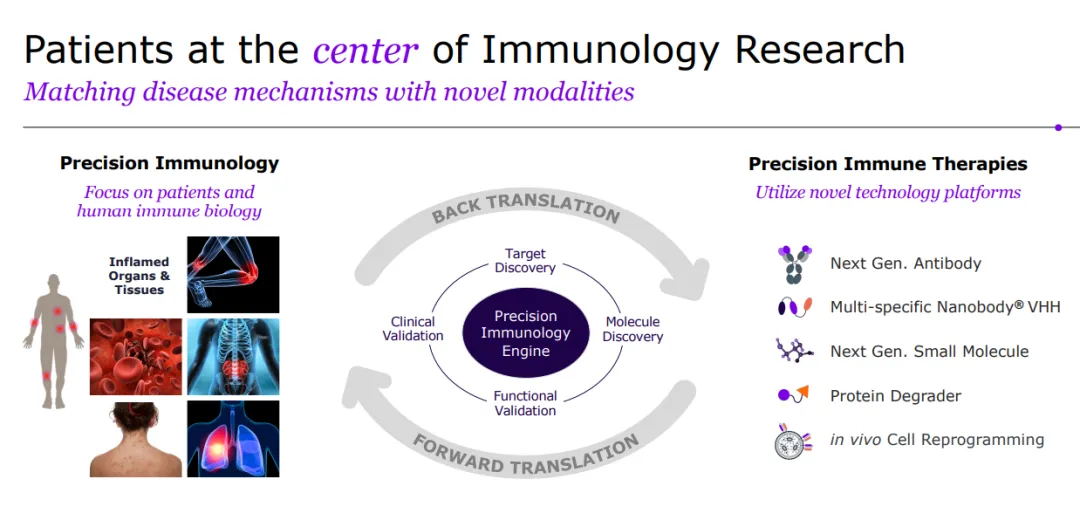
Sanofi is a global leader in immunology, dedicated to reshaping and prioritizing its immunology pipeline through focused strategic decisions, leveraging its expertise to advance areas such as neuroinflammation and rare diseases.
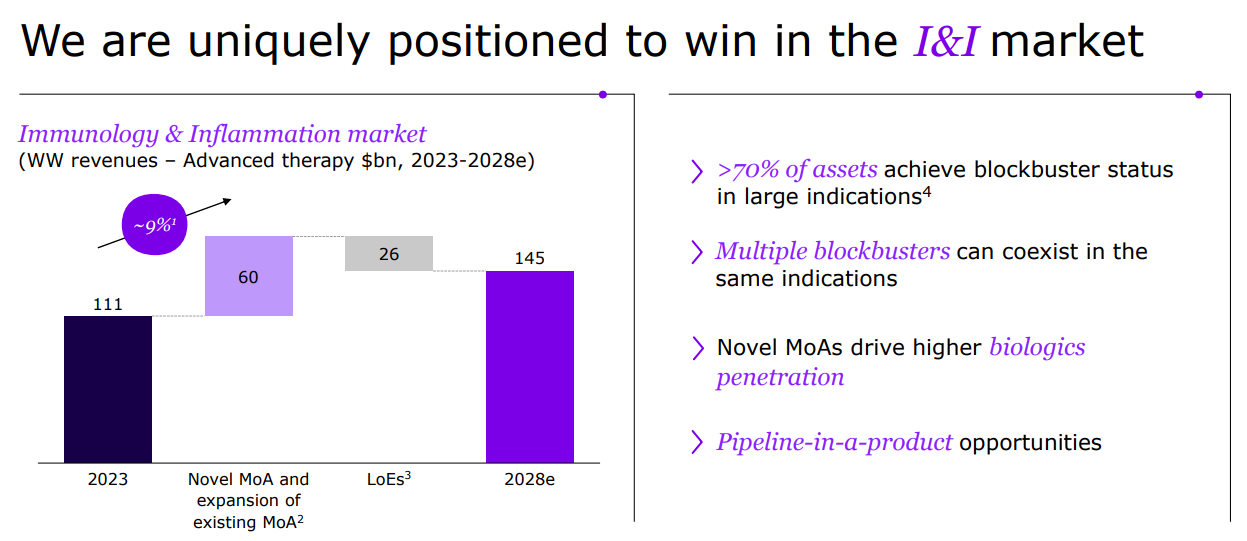
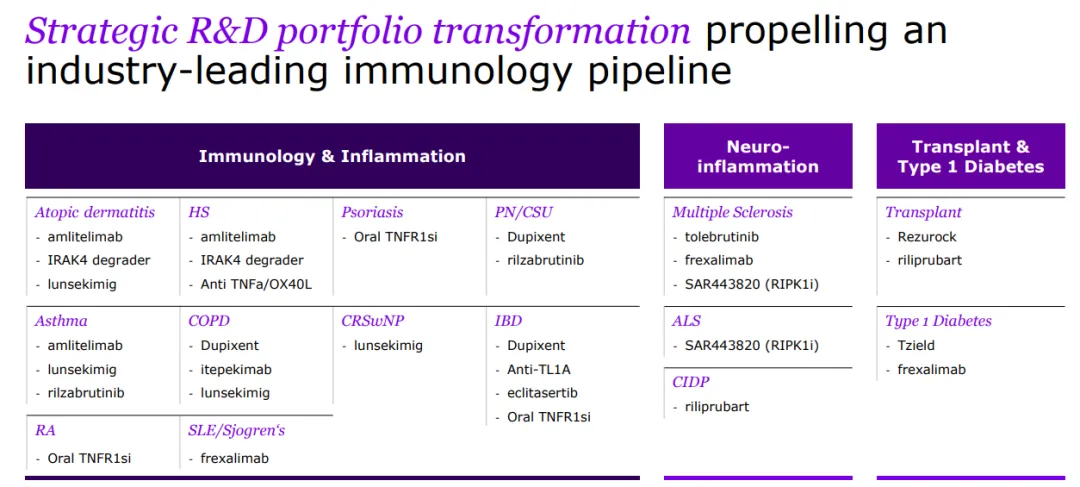
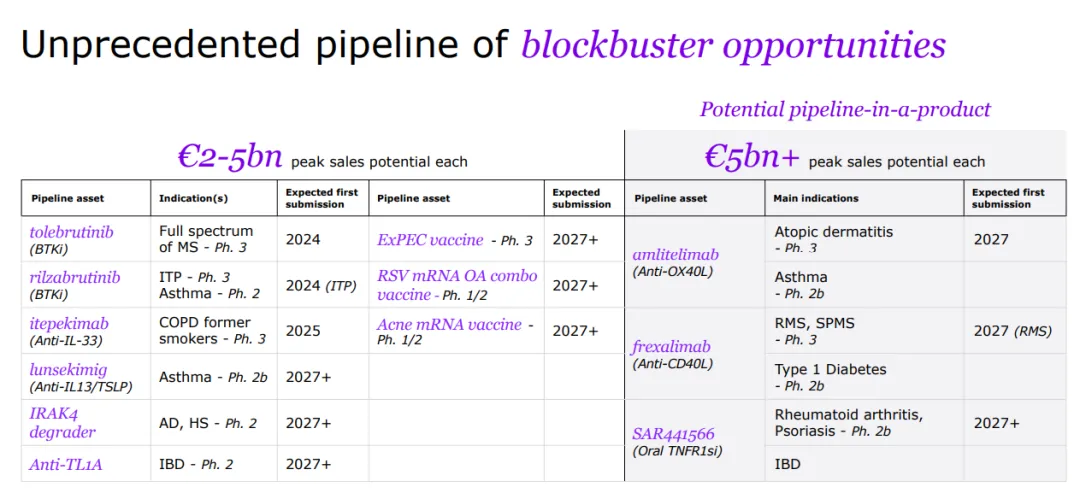
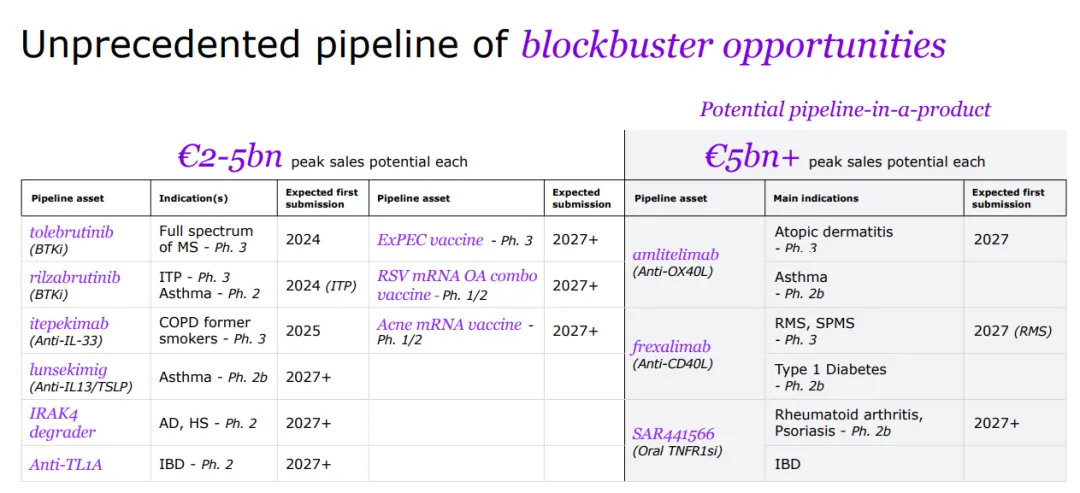
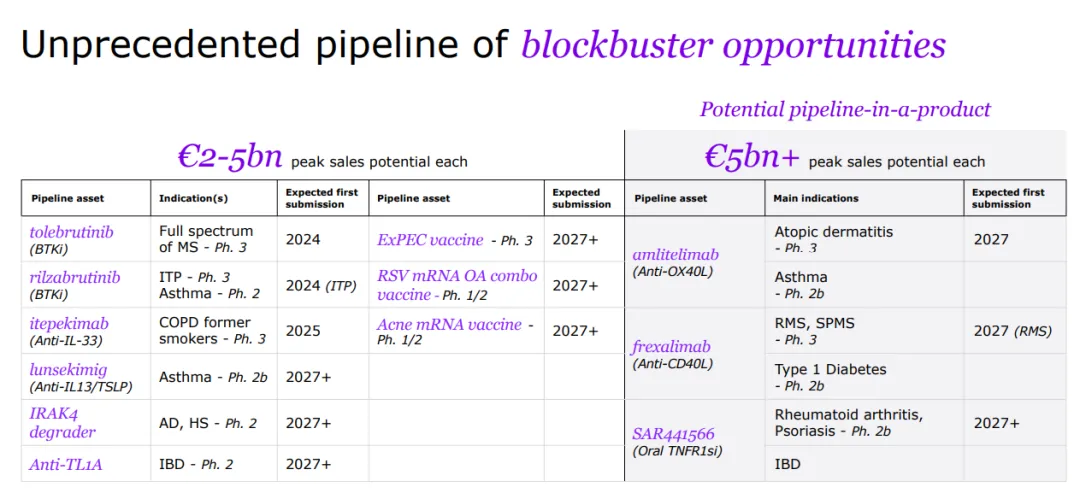
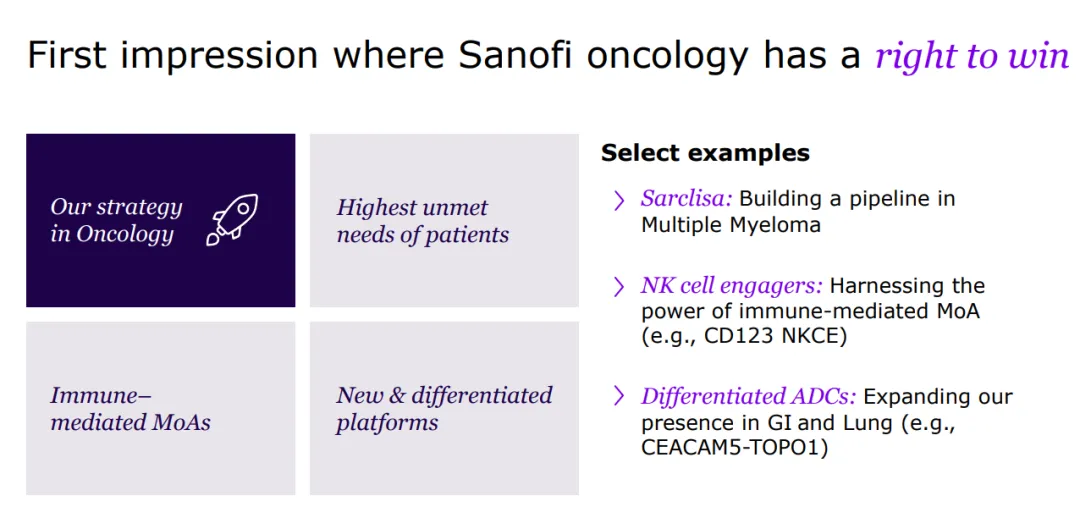
With a strong foundation in immunology, the company concentrates its expansion efforts on challenging-to-treat cancers like select hematological malignancies and solid tumors, and cancers with significant unmet needs, including multiple myeloma, acute myeloid leukemia, certain types of lymphoma, as well as gastrointestinal and lung cancers.

How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!