Satralizumab Unveiled: A Detailed Overview of its Revolutionary R&D Breakthroughs
Satralizumab's R&D Progress
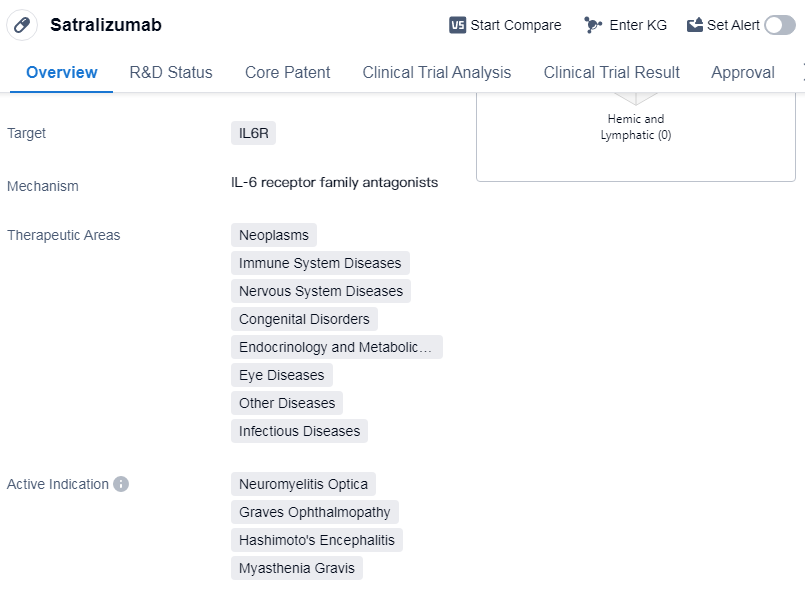
Satralizumab is a monoclonal antibody drug that targets IL6R, making it a potential treatment option for various diseases within the field of biomedicine. Its therapeutic areas include neoplasms, immune system diseases, nervous system diseases, congenital disorders, endocrinology and metabolic disease, eye diseases, other diseases, and infectious diseases. The drug is specifically indicated for the treatment of neuromyelitis optica, Graves ophthalmopathy, Hashimoto's encephalitis, and myasthenia gravis.
Satralizumab was developed by Roche Registration GmbH, a prominent organization in the pharmaceutical industry. It has reached the highest phase of development, with global approval obtained in June 2020. The drug has also received approval in China. The first country to approve Satralizumab was Canada.
In terms of regulation, Satralizumab has undergone various expedited review processes. It has been granted priority review, fast track designation, breakthrough therapy status, and orphan drug designation. These regulatory designations highlight the potential significance of Satralizumab in addressing unmet medical needs and accelerating its development and availability for patients.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Satralizumab: IL-6 receptor family antagonists
IL-6 receptor family antagonists are a class of drugs that specifically target and block the receptors of the interleukin-6 (IL-6) family. The IL-6 receptor family consists of various receptors, including the IL-6 receptor (IL-6R) and the glycoprotein 130 (gp130) receptor. These receptors play a crucial role in the signaling pathway of IL-6, a cytokine involved in inflammation and immune responses.
By antagonizing the IL-6 receptor family, these drugs inhibit the binding of IL-6 to its receptors, thereby preventing the downstream signaling cascade. This blockade ultimately reduces the inflammatory response mediated by IL-6, which can be beneficial in various conditions characterized by excessive inflammation.
IL-6 receptor family antagonists have shown efficacy in the treatment of several diseases, particularly autoimmune and inflammatory disorders. For example, they have been approved for the management of rheumatoid arthritis, juvenile idiopathic arthritis, giant cell arteritis, and Castleman's disease. These drugs help alleviate symptoms, reduce inflammation, and improve patients' quality of life.
It is important to note that IL-6 receptor family antagonists should be used under medical supervision due to potential side effects and contraindications. Common side effects may include infections, infusion reactions, and gastrointestinal disturbances. Therefore, patients receiving this type of treatment should be closely monitored by healthcare professionals.
Drug Target R&D Trends for Satralizumab
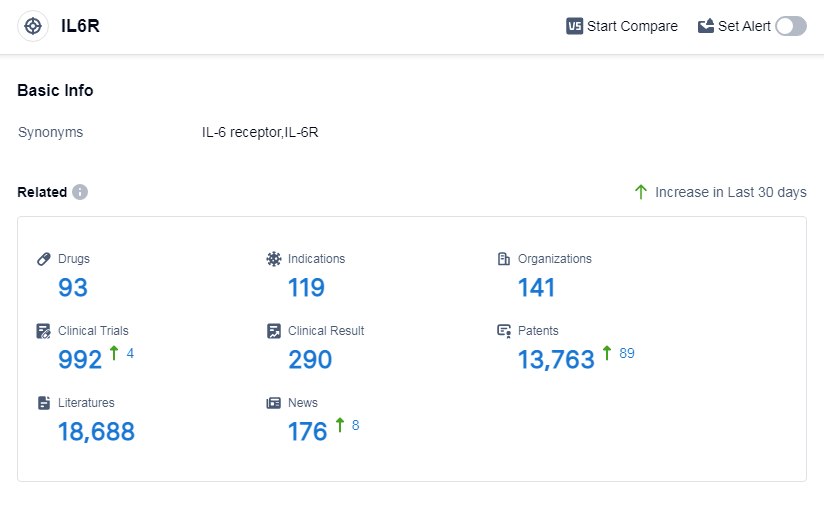
According to Patsnap Synapse, as of 14 Sep 2023, there are a total of 93 IL6R drugs worldwide, from 141 organizations, covering 119 indications, and conducting 992 clinical trials.
Based on the analysis of the data provided, Roche Holding AG is the company with the highest number of drugs under the IL6R target. The most common indications for approved drugs are thrombocytopenia, rheumatoid arthritis, COVID-19, cytokine release syndrome, and systemic onset juvenile chronic arthritis. Interleukins, monoclonal antibody, and biosimilar are the most common drug types in development. China, Japan, the United States, and the European Union are the countries/locations with the highest number of approved drugs under the IL6R target. The IL6R target shows a competitive landscape with multiple companies and drug types involved in its development. Future development in this target may focus on expanding indications and exploring new drug types.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
The objective presentation of this information provides a comprehensive overview of Satralizumab, its target, therapeutic areas, active indications, originator organization, approval status, and regulatory designations. This summary serves as a factual representation of the drug's key details, allowing readers to gain a clear understanding of its potential applications and significance in the pharmaceutical industry.