selectION Announces Phase 1b Trial Start for si-544 in Psoriasis Vulgaris and Psoriatic Arthritis Patients
selectION, Inc., a biopharmaceutical company in the clinical stage focusing on innovative therapies for T cell-mediated autoimmune disorders, declared the commencement of a Phase 1b study involving its leading candidate si-544 in adult individuals suffering from psoriasis vulgaris or psoriatic arthritis.
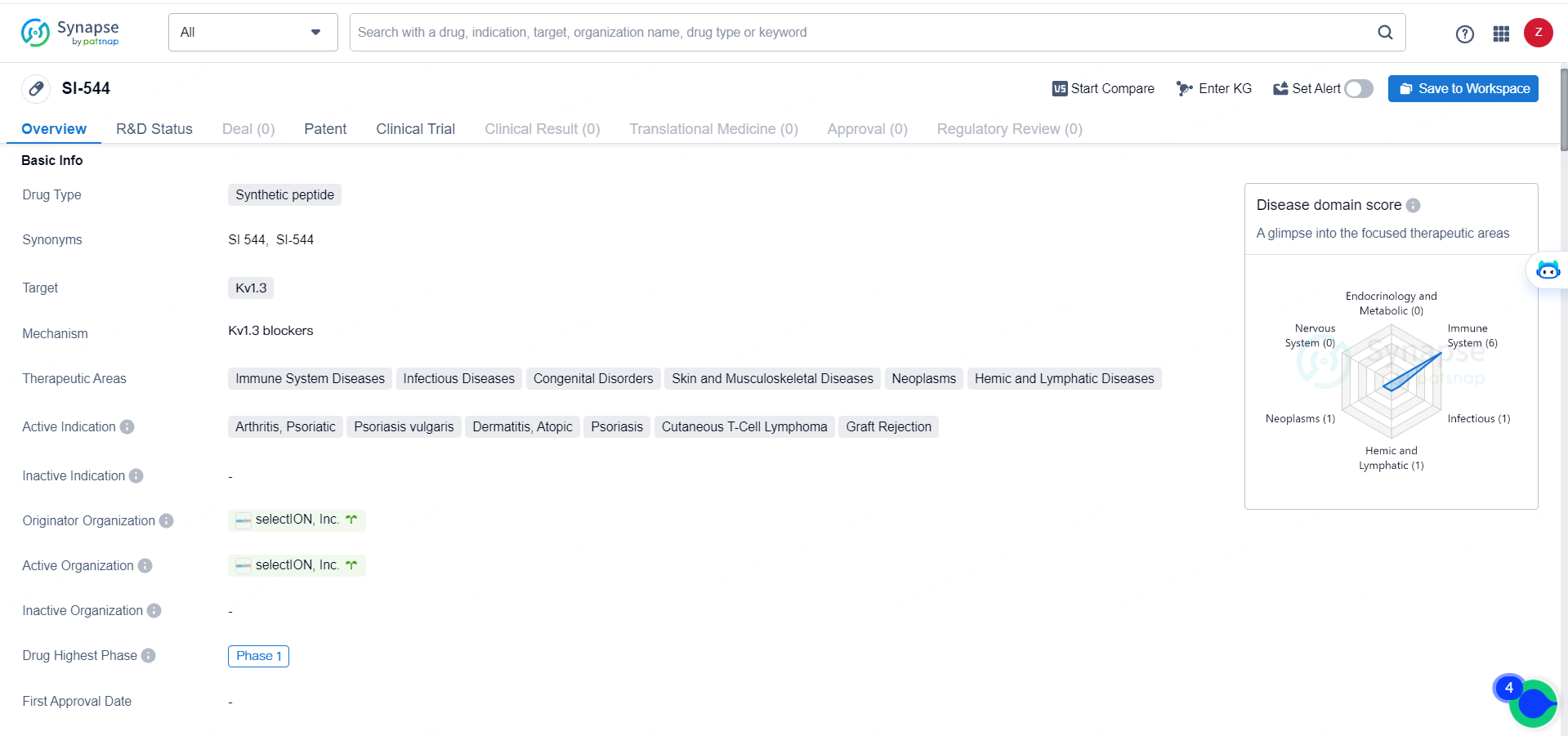
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
si-544 is a peptide designed for high selectivity in inhibiting the Kv1.3 ion channel. Kv1.3 represents a critical target in autoimmune diseases, and the Company believes si-544 could establish a new benchmark for safety and tolerability in treating T cell autoimmunity. Patient enrollment is ongoing, with top-line data anticipated in Q4 2024.
This latest clinical study is structured as a multicenter, Phase 1b, double-blind, placebo-controlled trial targeting adults with mild to severe Ps or PsA. The Company plans to recruit up to 40 Ps patients, with as many as 16 of them also having PsA.
Dr. Andreas Klostermann, Chief Scientific Officer and co-founder, stated, “Our primary goal is to expand the clinical development of si-544 for autoimmune conditions, and we eagerly await the results from this second, larger study in T cell-mediated disorders.”
Dr. Antonius Schuh, Chairman and CEO of selectION, Inc., commented, “The positive results from our clinical trial in atopic dermatitis patients further support the potential of targeting disease-related, persistently activated T cells through Kv1.3 inhibition to offer safe and effective treatments for various autoimmune diseases.”
The Company’s leading drug candidate, si-544, inhibits Kv1.3, an ion channel crucial for the activation and proliferation of TEM cells, with what the Company deems to be class-leading selectivity. TEM cells are implicated in numerous autoimmune conditions like atopic dermatitis, psoriasis, rheumatoid arthritis, multiple sclerosis, and some rare cancers such as lymphomas.
si-544 has shown an excellent safety and tolerability profile, according to the Company, in a recently concluded Phase 1b trial with atopic dermatitis patients, also suggesting initial efficacy. Prior to this, si-544 demonstrated remarkable efficacy in preclinical models involving animal and human T cells.
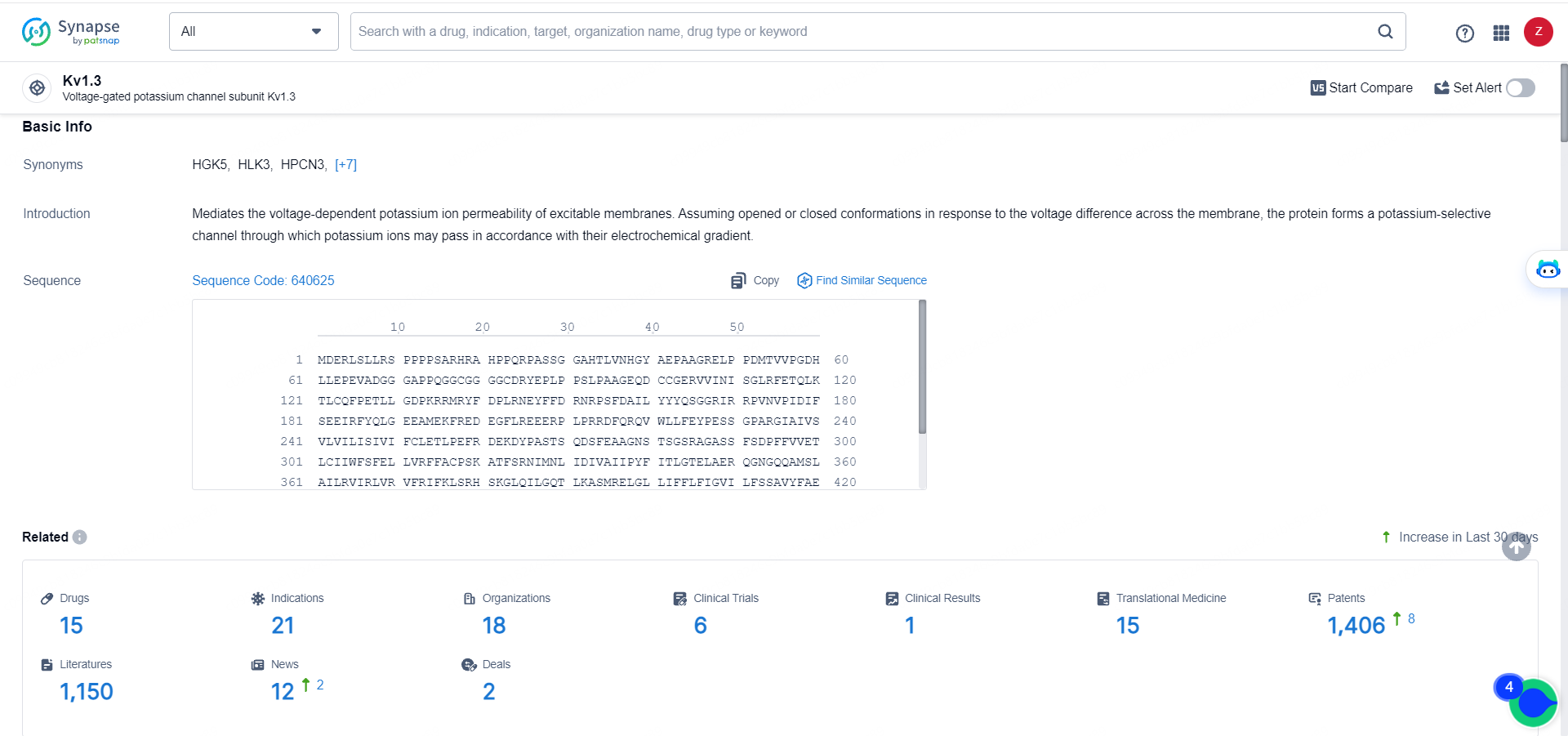
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of May 29, 2024, there are 15 investigational drugs for the Kv1.3 target, including 21 indications, 18 R&D institutions involved, with related clinical trials reaching 6, and as many as 1406 patents.
SI-544 is a synthetic peptide drug targeting Kv1.3, with a focus on addressing a wide range of diseases within the immune system, infectious diseases, and other related therapeutic areas. Its current status at Phase 1 indicates that it has shown promise in early human testing, and further development and evaluation are likely to continue.