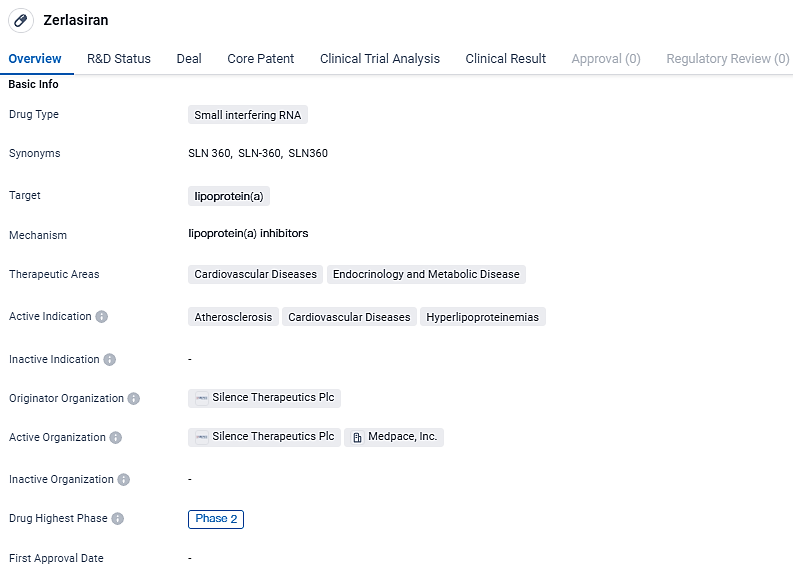
Silence Therapeutics' Zerlasiran Shows Promise in Phase 2 Trial for High Lipoprotein(a)
Silence Therapeutics plc, a leading biotech firm dedicated to improving patients' health by developing precisely tailored drugs that target and silence disease-causing mechanisms, disclosed encouraging initial 36-week results from the active ALPACAR-360 phase 2 trial involving zerlasiran (SLN360). The study includes 178 participants who have a starting baseline lipoprotein (Lp) measurement of 125 nmol/L or higher and are also at an elevated risk of experiencing events related to atherosclerotic cardiovascular disease.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Zerlasiran, an siRNA, is engineered to reduce the production level of Lp, a crucial genetic marker linked to cardiovascular ailments estimated to impact roughly one-fifth of the global populace.
Throughout a double-masked, placebo-managed phase, the administration of zerlasiran was carried out subcutaneously in dosages of 300 mg every 16 or 24 weeks, as well as 450 mg every 24 weeks, in individuals presenting an average initial Lp figure close to 215 nmol/L. The outcomes from this period showed a statistically profound drop in Lp levels from the initial baseline when measured against the placebo, enduring up to 36 weeks. Observations included a median Lp decrease of no less than 90% at 36 weeks for administered doses. Throughout this phase, no additional safety issues came to light.
The research, extending over 60 weeks, is still in progress. Researchers will analyze the secondary endpoints, such as changes in baseline Lp levels at weeks 48 and 60 and the possible influence on various fats and lipoproteins.
Steven Romano, MD, the chief of R&D at Silence, expressed optimism about the phase 2 findings, noting their high consistency with those from phase 1 and the potential they indicate for Zerlasiran's effectiveness in treating patients with elevated levels of Lp. Dr. Romano is looking forward to the forthcoming review of the data at 48 weeks and is hopeful about further development of Zerlasiran to combat the pressing health challenge posed by cardiovascular disease.
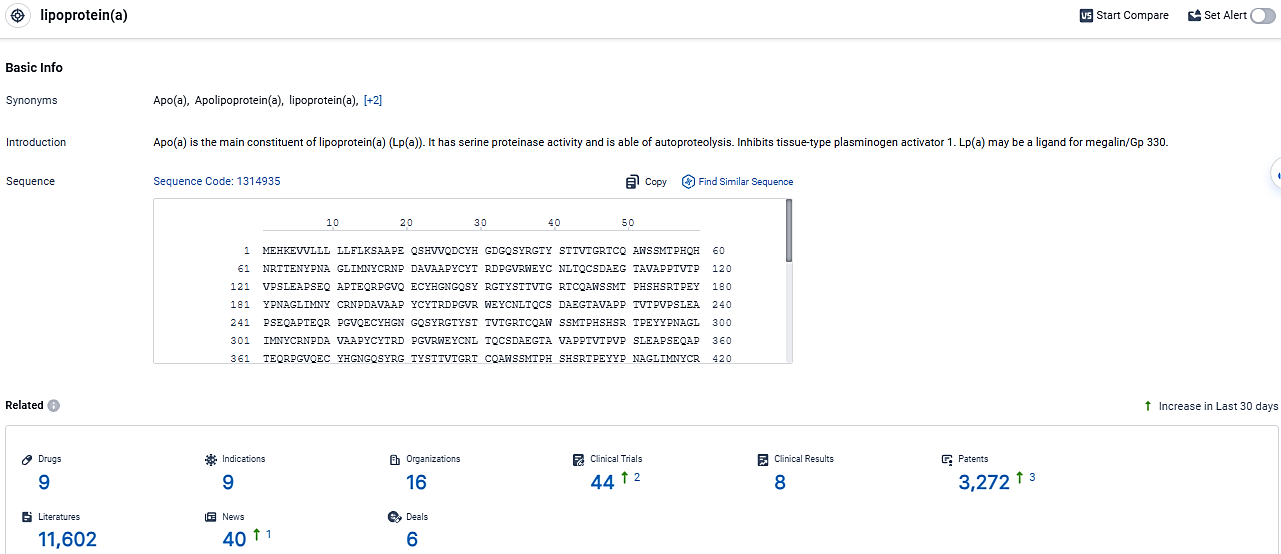
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of March 14, 2024, there are 9 investigational drugs for the lipoprotein target, including 9 indications, 16 R&D institutions involved, with related clinical trials reaching 44, and as many as 3272 patents.
Zerlasiran targets lipoprotein and aims to address cardiovascular diseases and hyperlipoproteinemias. Currently in Phase 2, Zerlasiran is being evaluated for its potential in treating atherosclerosis and related conditions.