Small RNA Drugs: Therapeutic Strategies, Sequence Analysis, and Chemical Modifications
RNA therapeutics, including small interfering RNA (siRNA), antisense oligonucleotides (ASO), microRNA (miRNA), small activating RNA (saRNA), messenger RNA (mRNA), and RNA aptamers, are becoming powerful tools for treating various diseases. These agents exert their effects through different mechanisms, including gene silencing, gene expression regulation, and interference with protein synthesis.
siRNA and ASO are two nucleic acid-based gene silencing technologies that achieve precise regulation of gene expression by complementary pairing with specific mRNA sequences. siRNA consists of double-stranded RNA that can be recognized by the RNA-induced silencing complex (RISC) within cells, guiding the complex to cleave target mRNA and preventing its translation into protein. This approach is widely used in gene function studies and therapeutic applications due to its efficiency and specificity. In contrast, ASOs are single-stranded DNA or RNA molecules that hinder translation or promote degradation of mRNA through complementary pairing. They offer better chemical stability and cellular penetration. ASOs employ various mechanisms, including blocking splicing, affecting mRNA stability, and activating RNAse H1. Despite differences in chemical structure and mechanisms, both siRNA and ASO aim to regulate gene expression, providing powerful tools for studying gene function and treating related diseases.
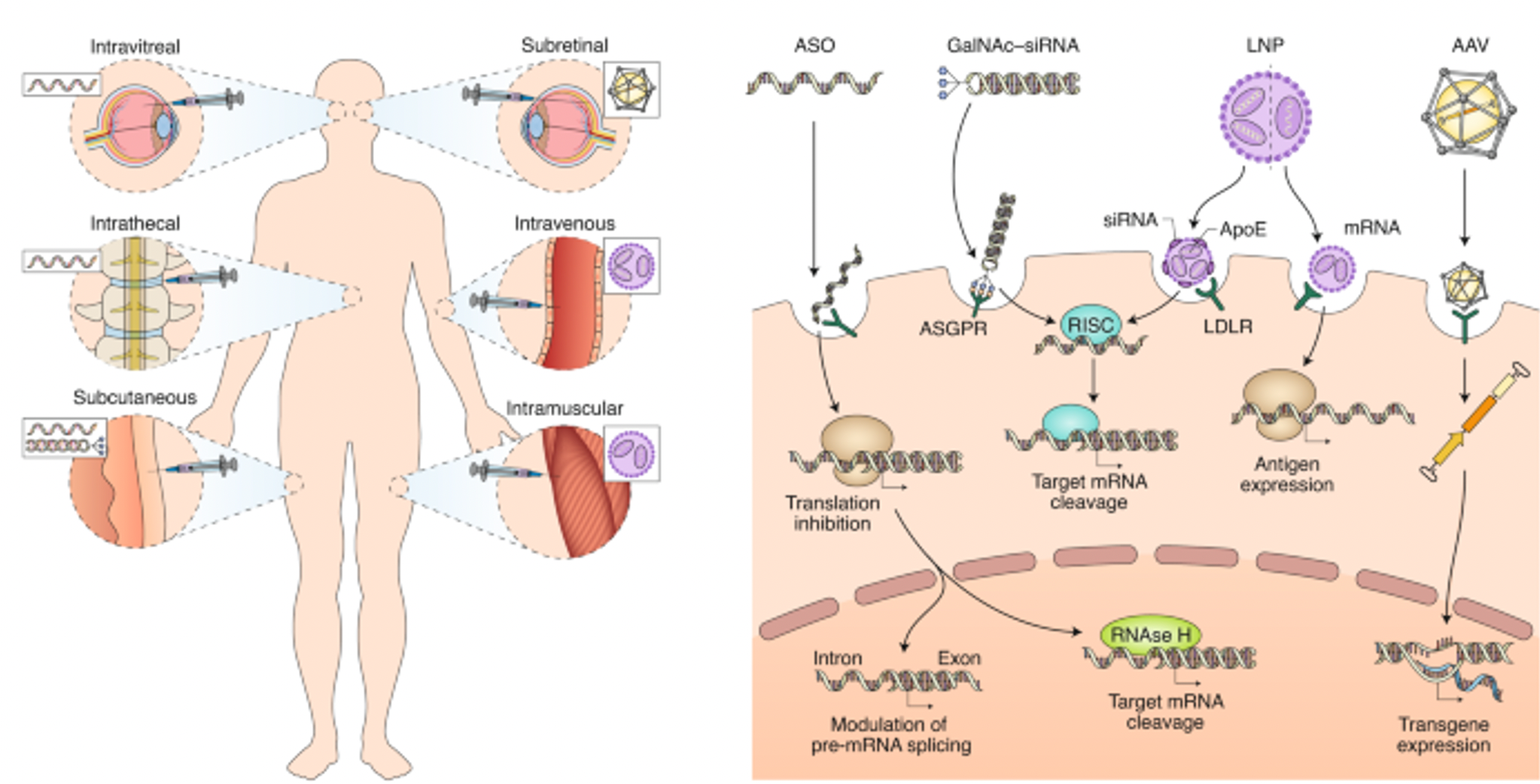
The core of designing small nucleic acid drugs lies in the precise selection of sequences and strategies for chemical modification. Sequence design is crucial for ensuring drug specificity, determining whether the drug molecule can effectively and specifically pair with target mRNA to achieve precise regulation of specific gene expression. Correct sequence selection can maximize drug efficacy while minimizing non-specific binding and potential off-target effects.

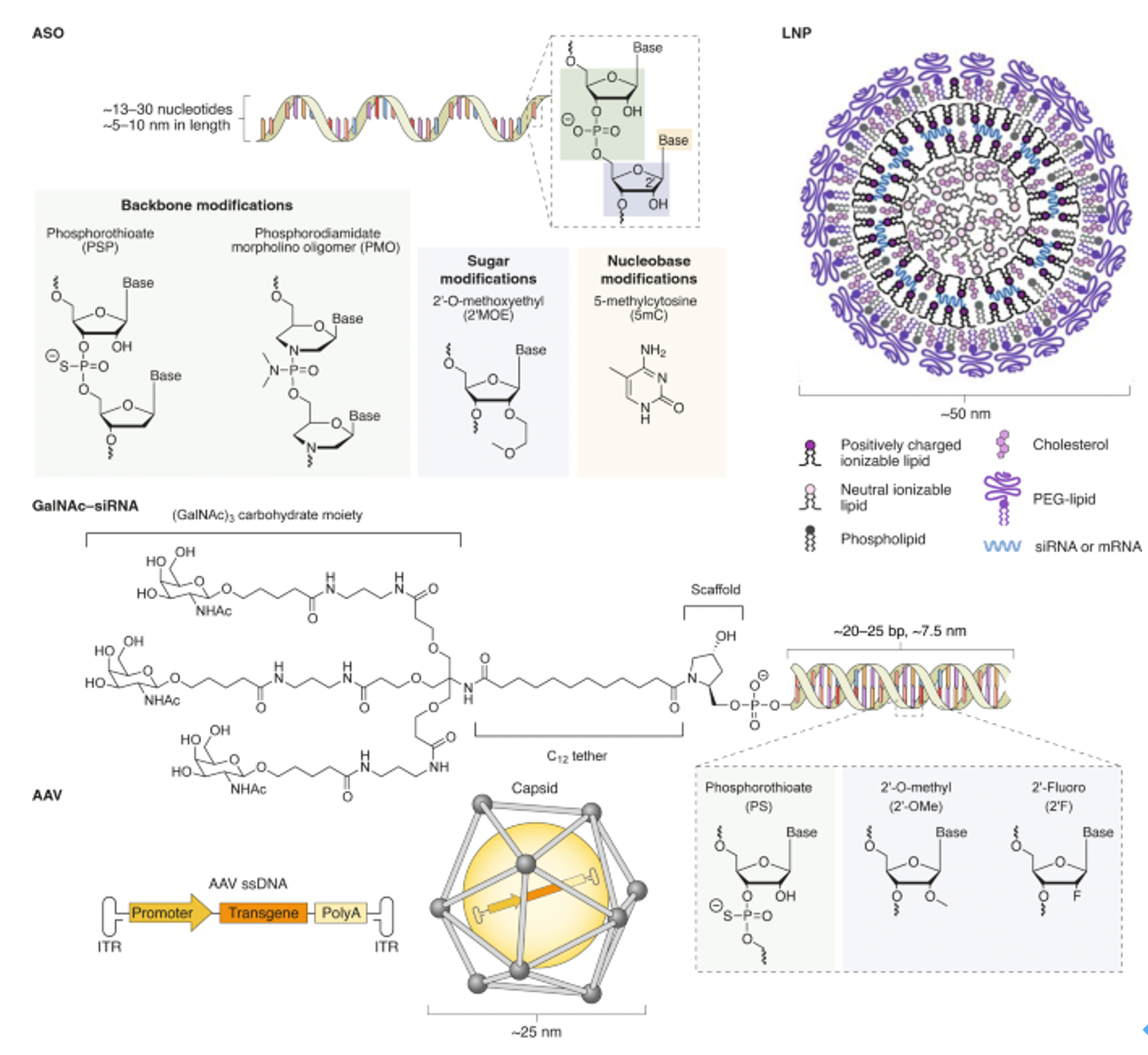
Chemical modifications are vital for enhancing the stability of small nucleic acid drugs in biological systems, improving their efficacy, prolonging half-lives, and reducing immunogenicity. Common modifications include 2'-O-methylation, phosphorothioate linkages (PS), and lipid conjugations like cholesterol modifications. The combination of these modifications significantly enhances the stability and efficacy of small nucleic acid drugs in vivo, showcasing their immense potential in gene silencing and disease treatment.
Patsnap Bio provides researchers with a powerful tool for retrieving and analyzing sequence information on small nucleic acid drugs, including core patent information, chemical modifications, and sequence alignment. Through this database, researchers can gain insights into the molecular structures and functions of small nucleic acid drugs, supporting drug design, efficacy evaluation, and clinical application strategies. Additionally, the database aids in monitoring patent dynamics, mitigating intellectual property risks, ensuring compliance in R&D efforts, and paving the way for market entry and commercialization.
Next, we will explore two small nucleic acid drugs to see what information Patsnap Bio can provide for their development.
Comparison of ASO Drug Nusinersen and siRNA Drug Inclisiran
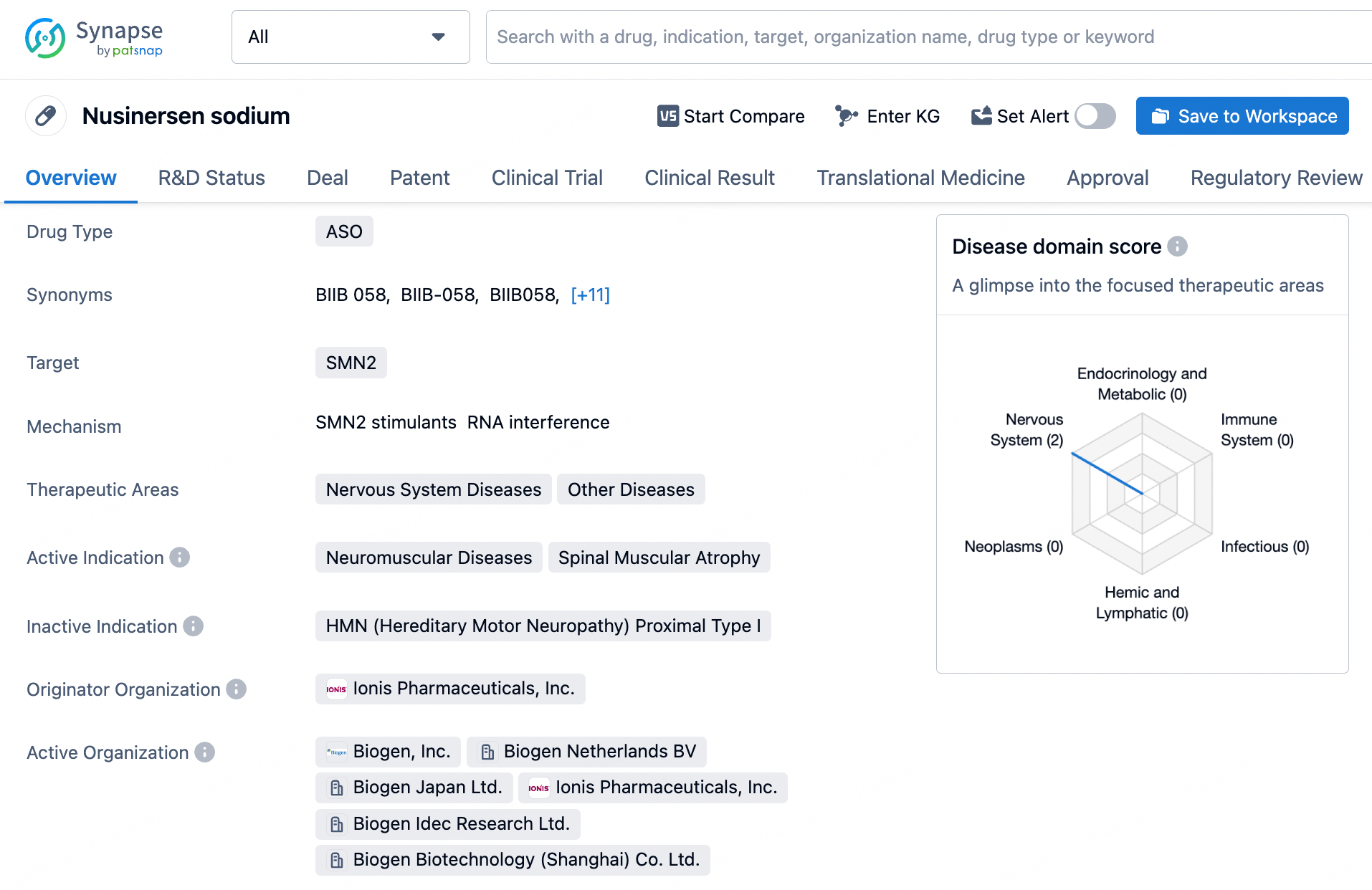
Nusinersen is an antisense oligonucleotide (ASO) developed by Biogen for the treatment of spinal muscular atrophy (SMA). SMA is a genetic neuromuscular disorder caused by mutations or deletions in the SMN1 gene, leading to the loss of motor neuron function. Nusinersen works by directly targeting the splicing of the SMN2 gene, thereby increasing the production of functional SMN protein, which alleviates the symptoms of SMA.
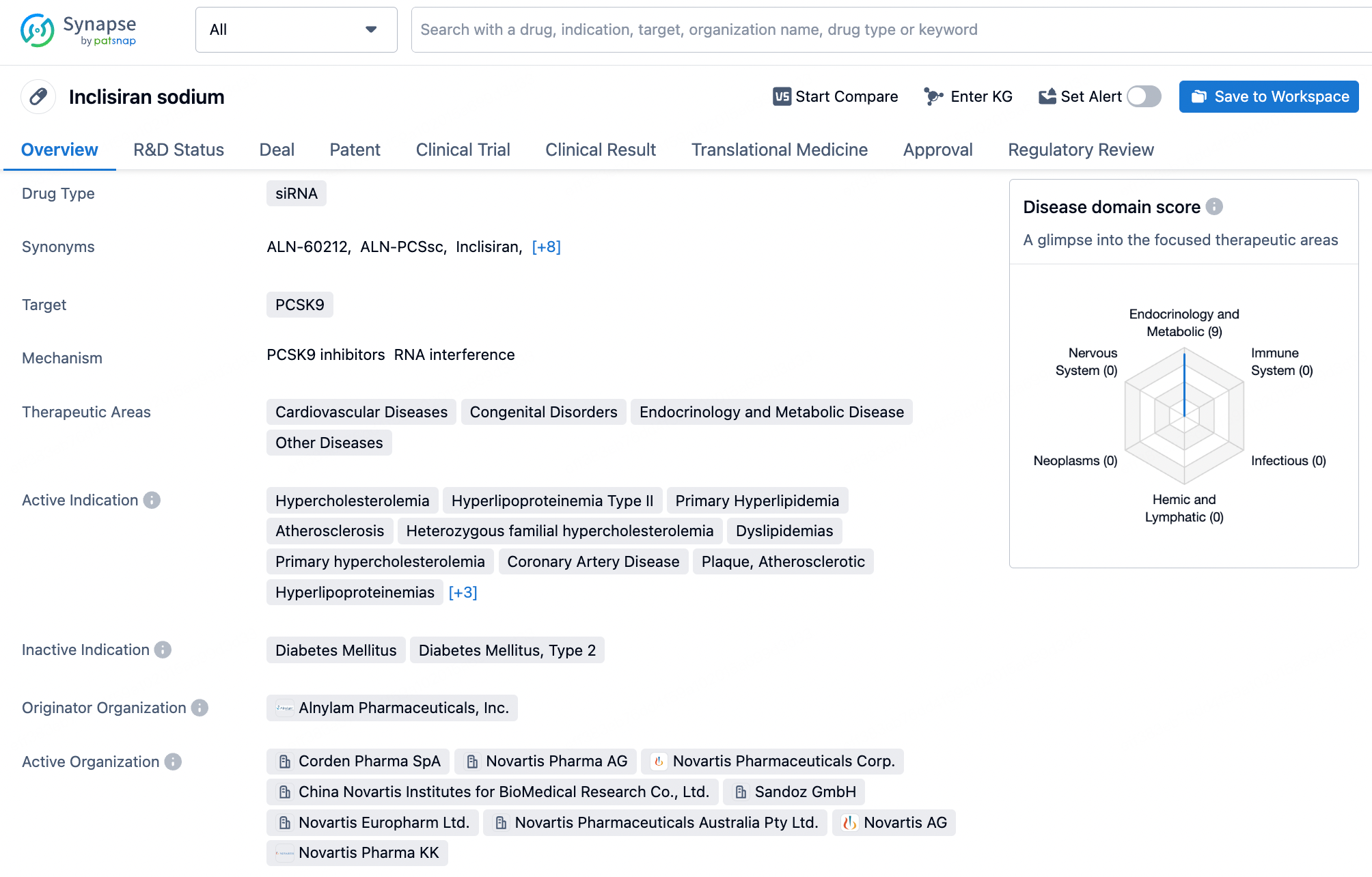
Inclisiran is a small interfering RNA (siRNA) drug developed by Alnylam Pharmaceuticals for the treatment of adult hypercholesterolemia and mixed dyslipidemia. Inclisiran targets the PCSK9 gene in the liver, reducing its expression and consequently decreasing the amount of PCSK9 protein produced by the liver. The PCSK9 protein decreases the liver's ability to clear low-density lipoprotein cholesterol (LDL-C) from the bloodstream. By lowering PCSK9 levels, Inclisiran aids in increasing the clearance of LDL-C, thereby reducing its levels in the blood.
Sequence Characteristics of Nusinersen and Inclisiran
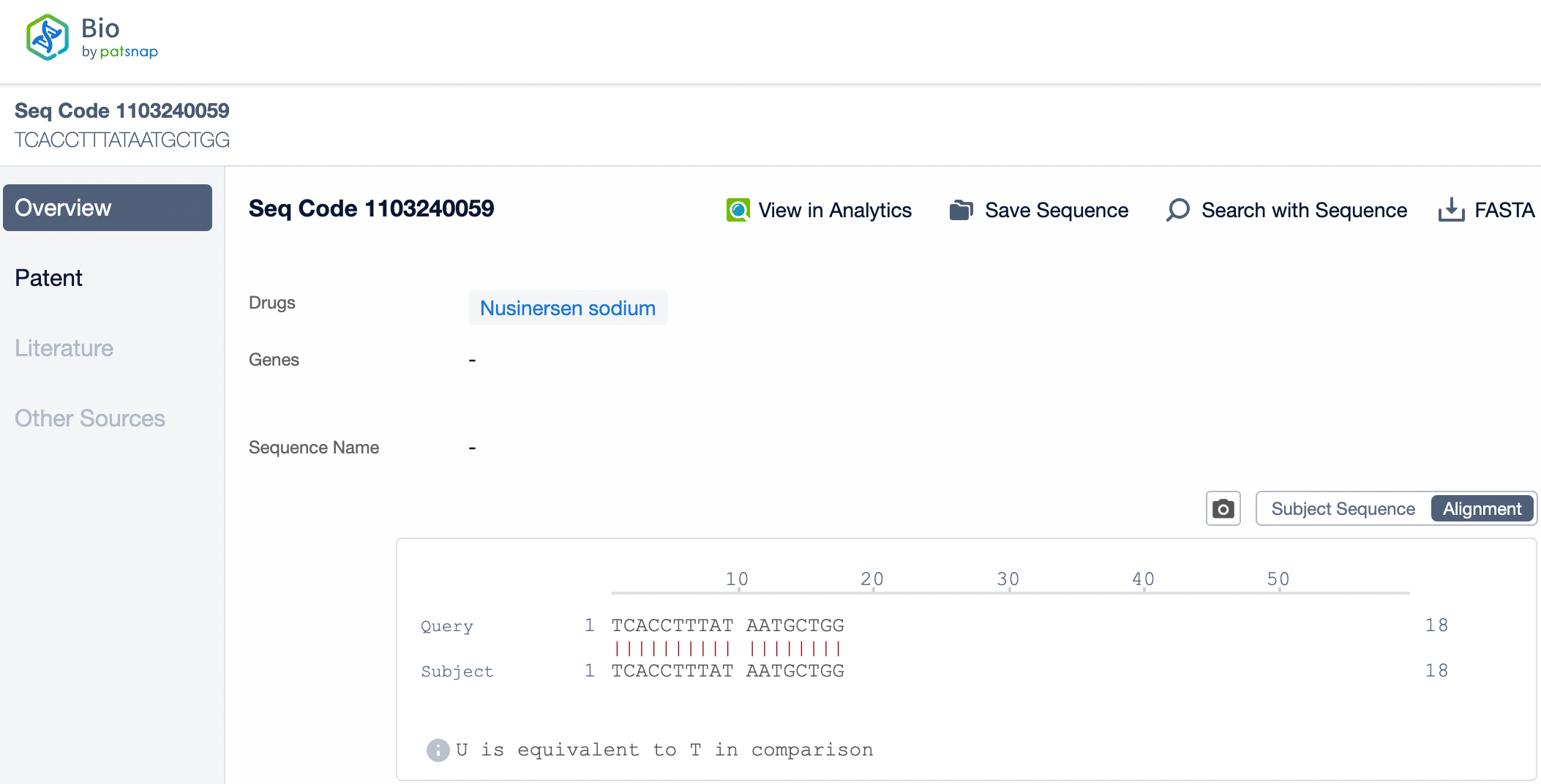
Nusinersen is a single-stranded DNA molecule composed of 18 nucleotides. It specifically binds to a particular sequence of the mRNA produced by the SMN2 gene in patients with spinal muscular atrophy (SMA) through complementary pairing. This binding alters the splicing pattern, promoting the inclusion of exons, a crucial step in the mRNA splicing of the SMN2 gene, which leads to an increase in functional SMN protein. Due to the high specificity of Nusinersen, it can selectively target SMN2 mRNA, reducing nonspecific binding to other non-target mRNAs and thereby lowering the risk of off-target effects.
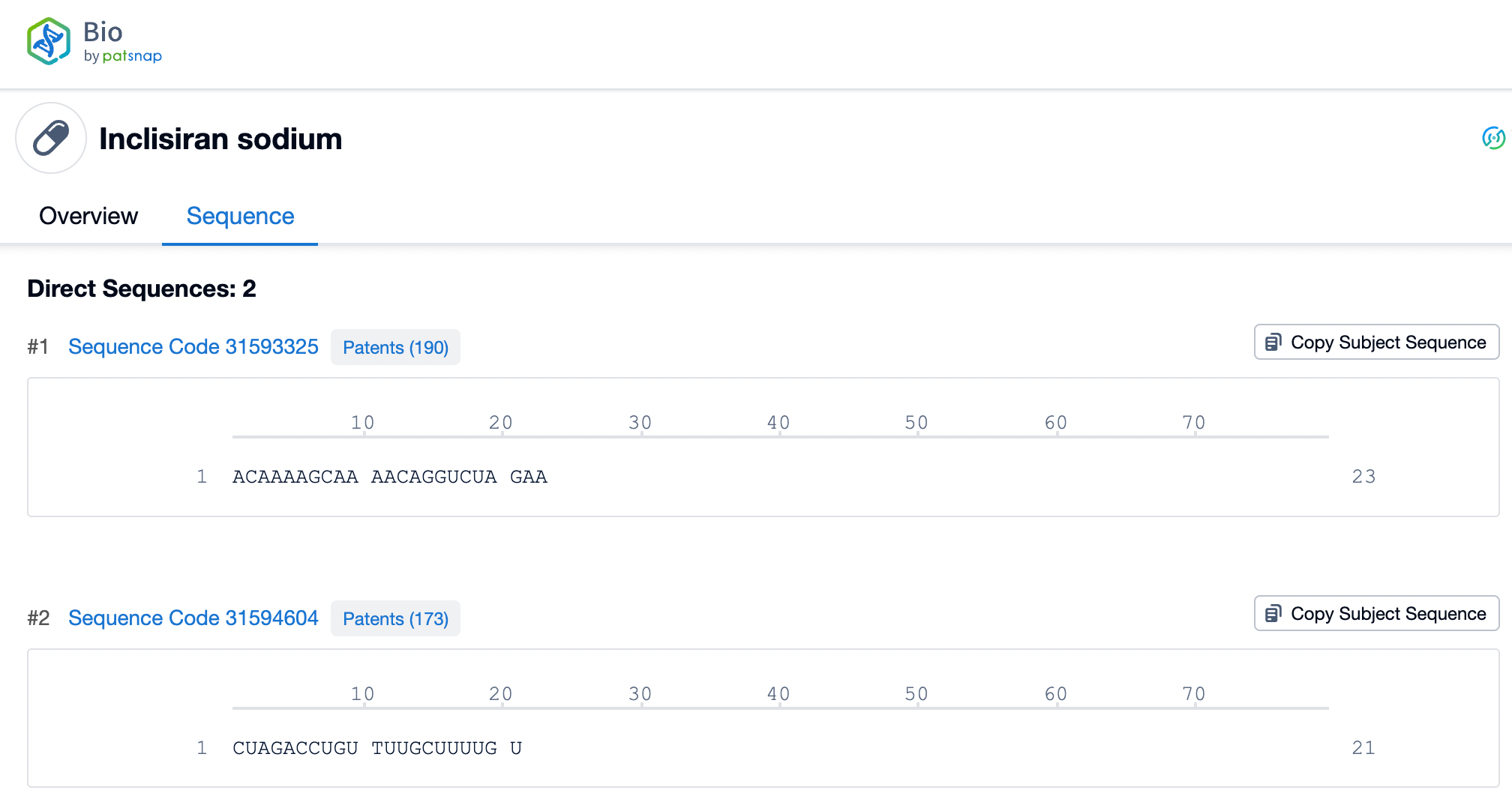
Inclisiran, as an innovative siRNA therapy, has a double-stranded RNA structure that is central to its therapeutic potential. The sense strand of Inclisiran consists of 21 nucleotides, while the antisense strand comprises 23 nucleotides. These two RNA strands are tightly bound through precise base pairing, forming a stable double helix structure. This structure enables Inclisiran to specifically bind to mRNA molecules within liver cells. Through this binding, Inclisiran triggers the RNA interference (RNAi) mechanism, specifically targeting the mRNA of the PCSK9 gene, leading to its degradation and thus reducing the production of PCSK9 protein.
Chemical Modifications of Nusinersen and Inclisiran
Nusinersen consists of 18 nucleotides and is designed as a gapmer structure, characterized by an unmodified deoxynucleotide region in the center, flanked by nucleotide segments modified with 2'-O-methyl (2'-MOE) groups at both ends. These modifications enhance the drug's stability and increase its affinity for the target mRNA. Additionally, all cytosine bases in Nusinersen have a methyl group introduced at the 5' position. This modification helps reduce potential immune stimulation side effects caused by the drug, particularly in single-stranded antisense oligonucleotides (ASOs). Methylation can decrease binding to Toll-like receptor 9 (TLR9), thereby minimizing potential immune activation. These carefully designed chemical modifications allow Nusinersen to effectively penetrate cell membranes, specifically bind to the splicing sites of SMN2 mRNA, and guide correct splicing, ultimately increasing the production of functional SMN protein to counteract the pathological processes of SMA.
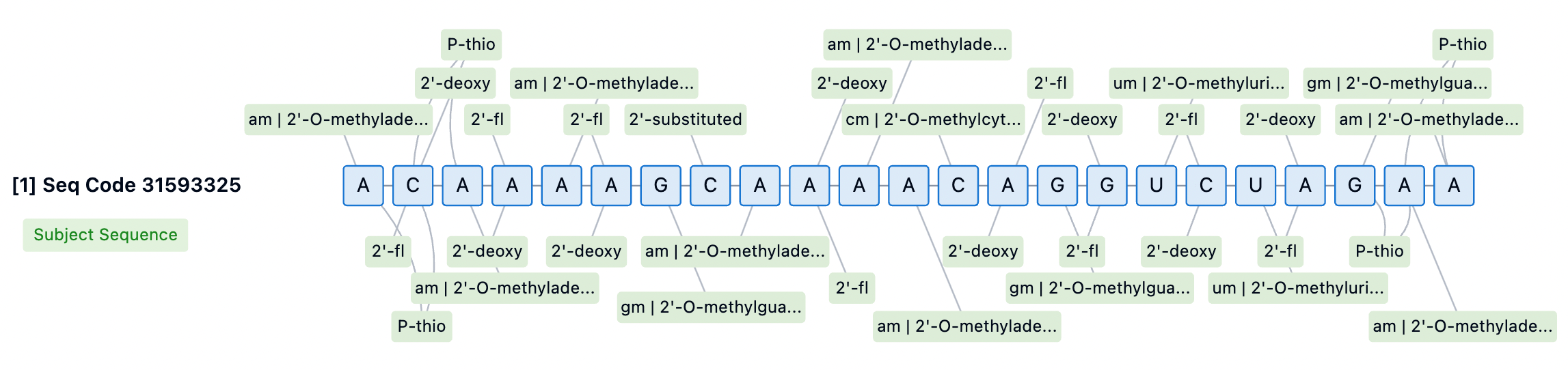
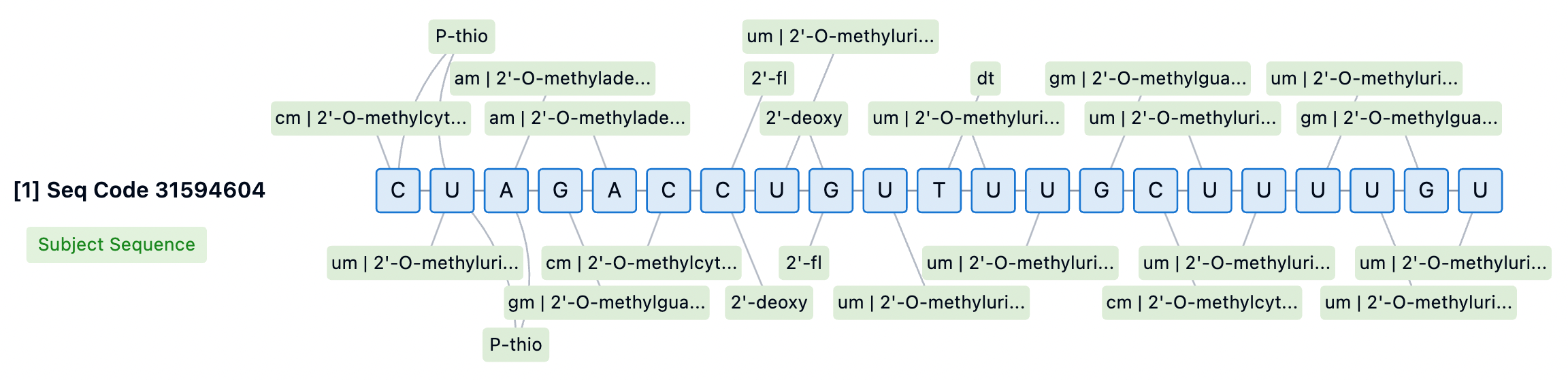
Inclisiran's sense strand is linked at the 5' end through a GalNAc conjugate (L96), a glycosylation modification that significantly enhances the cellular uptake and endocytosis of the siRNA in the liver. The chemical modifications of Inclisiran also include 2'-fluoro (2'-F) and 2'-O-methyl (2'-OMe) substitutions on the ribose ring, which help protect the siRNA from degradation by nucleases in serum, providing a longer circulation time in vivo. Moreover, several phosphodiester bonds in the siRNA molecule have been replaced with phosphorothioate (PS) modifications, further enhancing the stability of the molecule and potentially reducing immune stimulation responses.
Summary
Small nucleic acid drugs, such as siRNA and ASO, are gradually becoming powerful tools for treating various diseases, providing new therapeutic strategies for modern medicine through precise gene silencing or expression regulation mechanisms. Nusinersen and Inclisiran exemplify the applications of ASO and siRNA technologies, respectively. The design and modification strategies of these drugs, such as 2'-O-methylation and phosphorothioate bonds, are aimed at enhancing stability, extending half-life, and reducing immunogenicity, thereby optimizing their therapeutic efficacy and safety.
Patsnap Bio offers researchers a valuable resource for in-depth analysis of the sequence characteristics and chemical modifications of these small nucleic acid drugs, which is crucial for understanding their molecular mechanisms and clinical applications. Through this database, researchers can access key sequence information, including core patent data and generic sequence comparisons, facilitating more informed decisions in drug design, efficacy assessment, and clinical strategy formulation. Additionally, the database supports patent monitoring to avoid intellectual property risks, ensuring compliance in research and development activities and providing support for drug market access and commercialization. As the field of small nucleic acid drugs continues to expand, Patsnap Bio will become an essential tool for driving innovation and development in this area.
Better answers for better bio-innovations!
Validate novelty, eliminate risk, and innovate with confidence using the world’s largest sequence database curated from millions of patent and non-patent sources.
Patsnap Bio helps you turn weeks into minutes with cutting-edge AI-enabled tools built to master the complexities of sequence retrieval and automate IP analysis with precision and ease.
With best-in-class coverage of protein and nucleic acid sequences combined with state-of- the-art search algorithms, you’ll spend less time searching and more time bringing your bio-innovations to market.
Reference
1.Kulkarni, J. A. et al. Author Correction: The current landscape of nucleic acid therapeutics. Nat Nanotechnol 16, 841 (2021). https://s10-doi-org.libproxy1.nus.edu.sg/s41565-021-00937-w
2.Paunovska, K., Loughrey, D. & Dahlman, J. E. Drug delivery systems for RNA therapeutics. Nat Rev Genet 23, 265-280 (2022). https://s10-doi-org.libproxy1.nus.edu.sg/s41576-021-00439-4




