Spyre Therapeutics Begins Phase 1 Trials for New Anti-TL1A Antibodies
Spyre Therapeutics, Inc. (NASDAQ: SYRE) is a biotechnology firm in the clinical development phase that focuses on advanced antibody engineering, strategic therapeutic combinations, and precision medicine to enhance the effectiveness and convenience of treating Inflammatory Bowel Disease (“IBD”). The Company has now commenced the administration of doses to healthy participants in Phase 1 clinical trials for two experimental half-life extended anti-TL1A monoclonal antibodies.
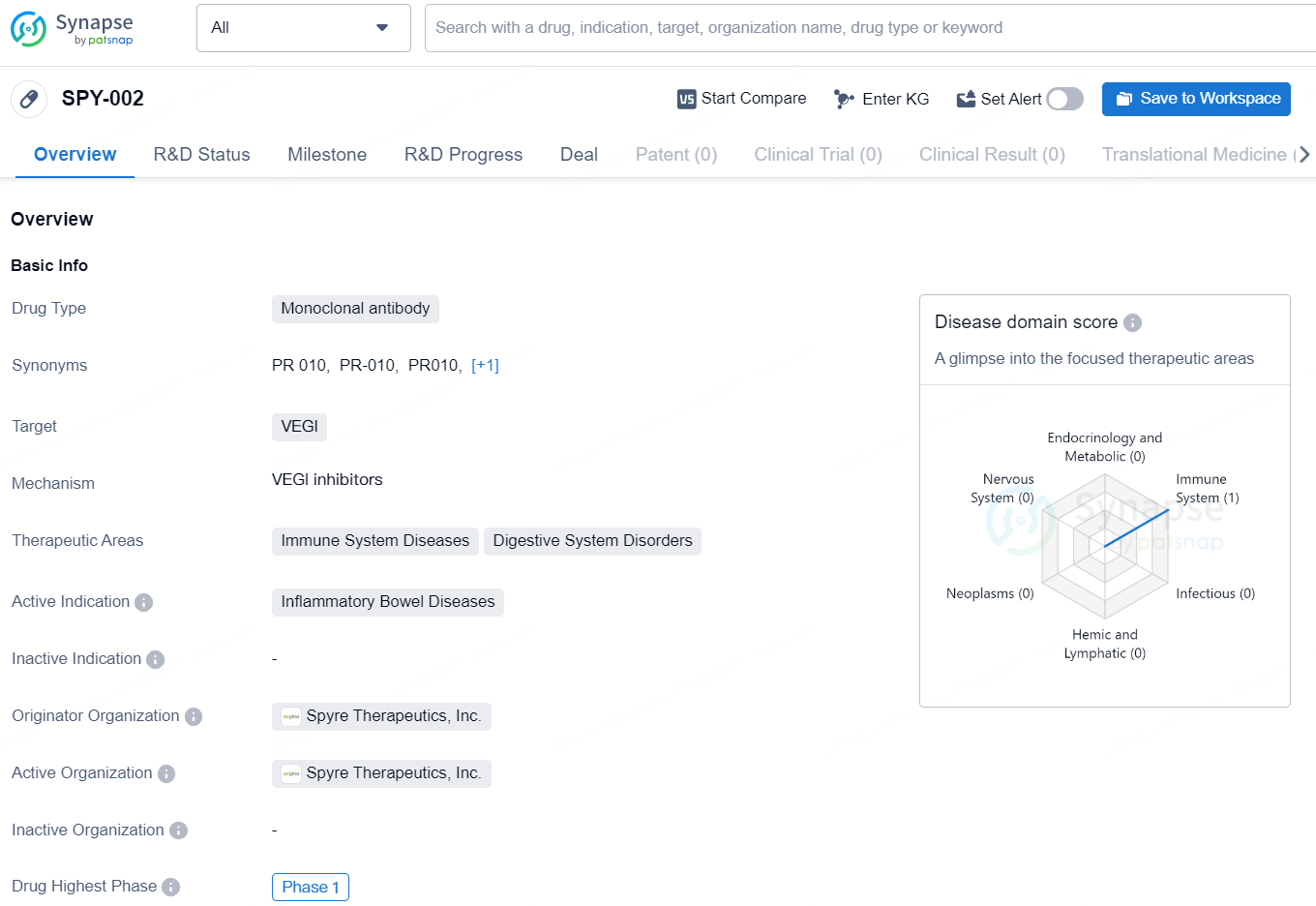
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
“TL1A inhibition has shown significant effectiveness in patients with ulcerative colitis and Crohn’s disease, and studies in pre-clinical IBD models indicate that it can enhance the effects of other targeted therapies when used together. Additionally, TL1A is associated with various inflammatory and fibrotic conditions outside of IBD,” stated Josh Friedman, M.D., Ph.D., Senior Vice President of Clinical Development at Spyre. “Our SPY002 compounds have been designed to leverage the findings from earlier molecules, featuring improved characteristics such as picomolar potency, longer half-lives, and concentrated formulations.”
The SPY002 Phase 1 Trials (NCT06672718 and NCT06622070) consist of double-blind, placebo-controlled studies involving single ascending doses administered to healthy subjects. Each trial aims to recruit around 56 healthy adult participants. The primary focus will be on safety, with pharmacokinetics (PK) analyzed as a secondary objective. Interim findings related to safety, PK, and pharmacodynamics (PD) from these studies are projected to be available in the second quarter of 2025. Based on the outcomes of the Phase 1 trials, the Company expects to advance the SPY002 program into Phase 2 by 2025.
“Commencing clinical trials with two optimized anti-TL1A compounds represents an exciting development as we build upon the promising results from Phase 1 for our next-generation anti-α4β7 antibody, SPY001, which has demonstrated a half-life exceeding 90 days, allowing for quarterly or biannual maintenance dosing. If Phase 1 results are favorable and we receive positive regulatory input, we anticipate launching one of the SPY002 candidates in our innovative Phase 2 study focusing on monotherapies and combination treatments for ulcerative colitis next year, along with beginning a streamlined Phase 2 proof-of-concept trial outside of IBD,” commented Cameron Turtle, D.Phil., Chief Executive Officer of Spyre. “Both studies are fully funded following our recent oversubscribed fundraising. The first clinical study for SPY003, our long-acting IL-23 antibody, is scheduled to commence in the first quarter of 2025, representing our fourth optimized antibody to begin clinical trials within a nine-month period.”
As of September 30, 2024, the Company reported a pro forma cash balance of approximately $630.1 million, which consists of around $414.2 million in cash, cash equivalents, and marketable securities, along with roughly $215.9 million in net proceeds from a previously disclosed underwritten public offering where underwriters fully exercised their option to acquire additional common stock, assuming no other financial changes have occurred since that date.
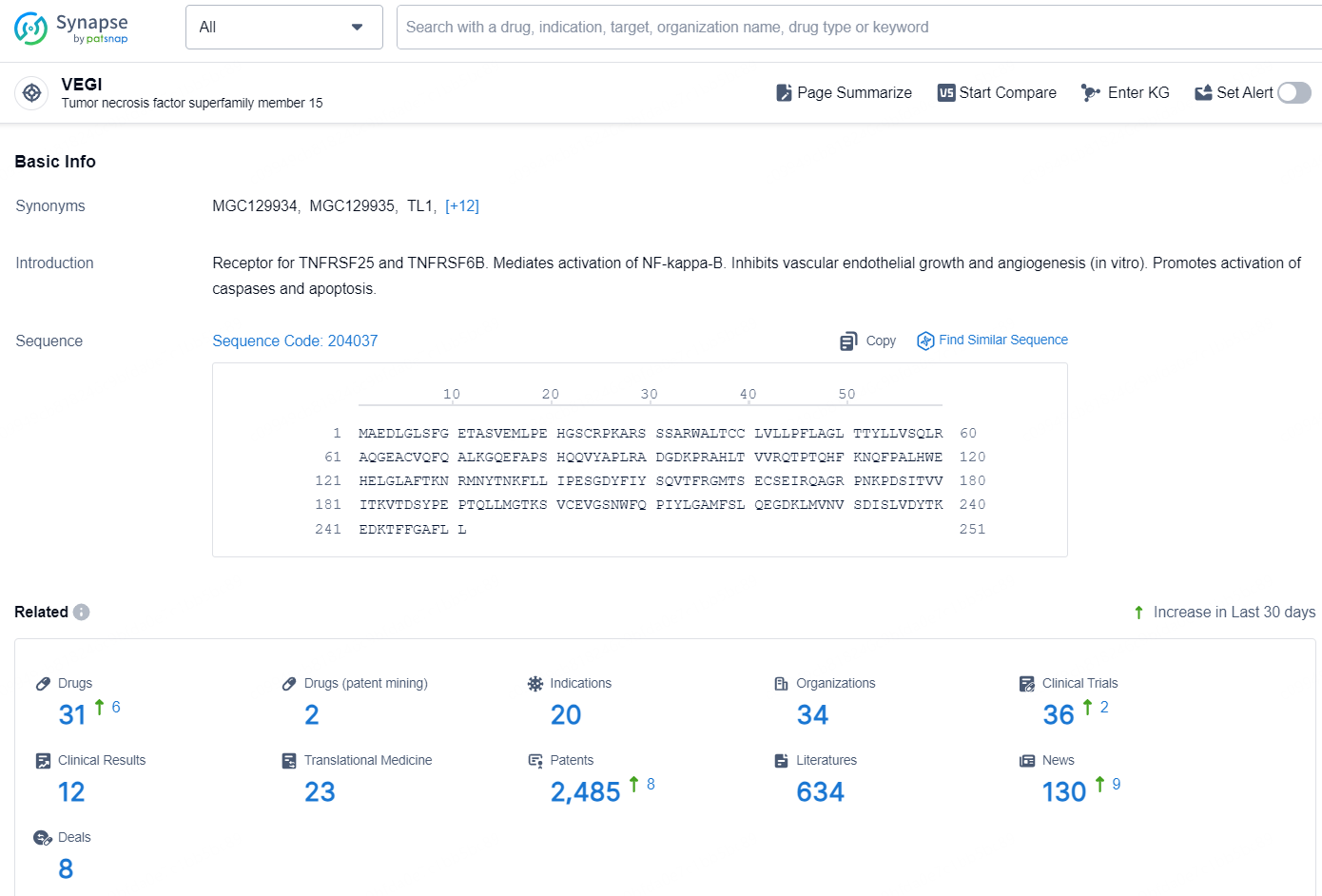
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of December 6, 2024, there are 31 investigational drugs for the VEGI target, including 20 indications, 34 R&D institutions involved, with related clinical trials reaching 36, and as many as 2485 patents.
SPY-002 is a monoclonal antibody drug developed by Spyre Therapeutics, Inc. The drug targets VEGI and is primarily focused on treating immune system diseases and digestive system disorders. Its active indication is for the treatment of inflammatory bowel diseases. As of the latest available information, SPY-002 has reached the Phase 1 stage of development globally.