Theriva™ Biologics Announces Positive Review by Data and Safety Committee in Early Stage Trial of SYN-004 for Transplant Patients
Theriva™ Biologics, Inc. (NYSE American: TOVX), is a versatile clinical-stage enterprise focused on creating treatments for cancer and related diseases in areas with significant unmet needs. The Company reported favorable conclusions from the Data and Safety Monitoring Committee (DSMC) review concerning the outcomes from the second cohort of its Phase 1b/2a randomized, double-blind, placebo-controlled clinical trial of SYN-004 (ribaxamase). This trial is conducted among recipients of allogeneic hematopoietic cell transplants (HCT) to prevent acute graft-versus-host disease (aGVHD).
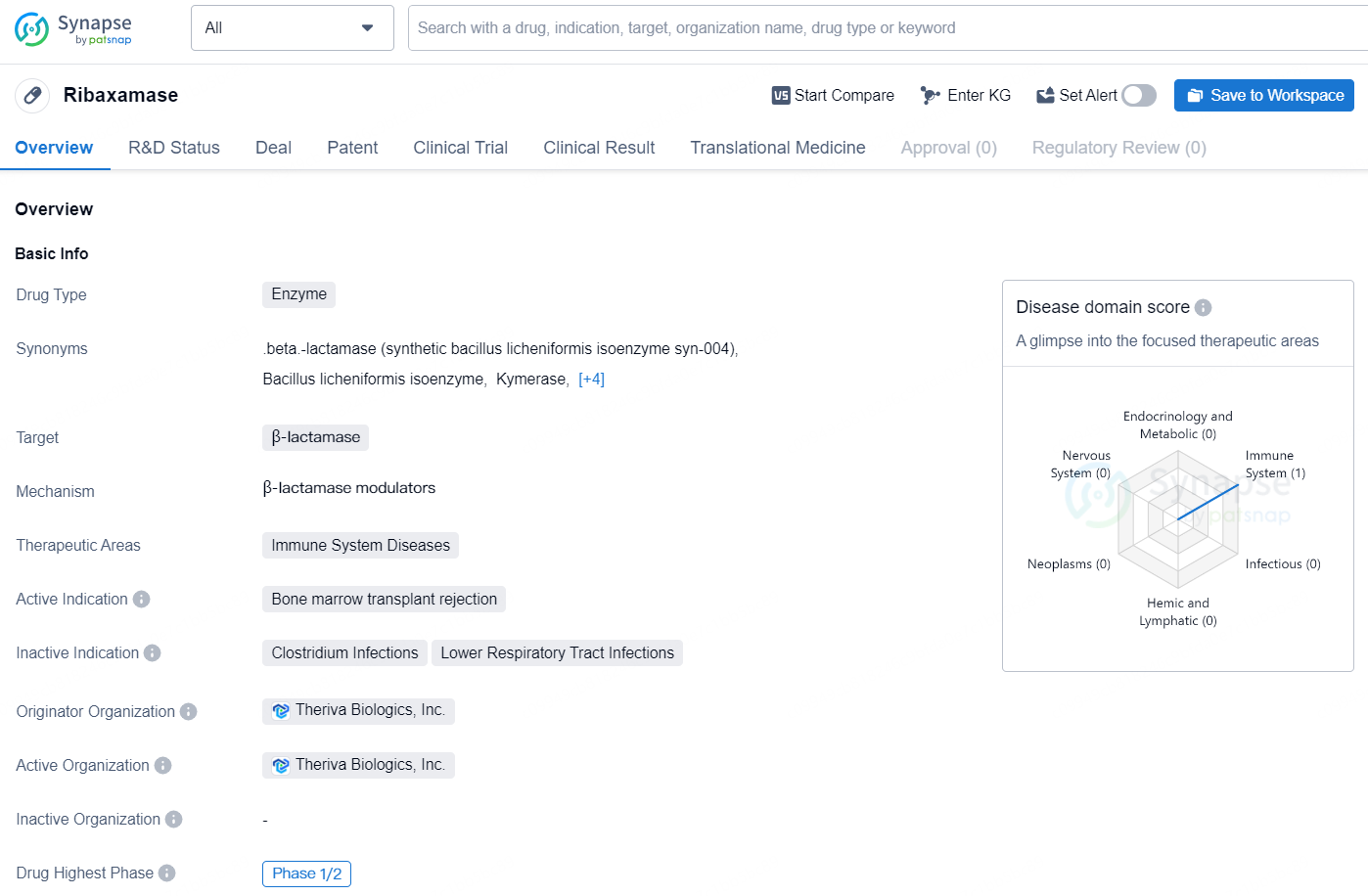
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
In Cohort 2, 19 patients were enrolled and administered at least one dose of the study medication (either SYN-004 or placebo in a 2:1 randomization). Among these, 18 participants received intravenous (IV) piperacillin/tazobactam at least once, and 12 patients completed a sufficient number of doses to be assessed for the study's endpoints. The study is currently still active and blind, but key insights from anonymized data for Cohort 2 are presented below:
Adverse events (AEs) and serious adverse events (SAEs) documented in Cohort 2 were typical for patients who have undergone allo-HCT, with no AEs or SAEs linked by investigators to the study drug treatment.
Fifteen SAEs were recorded across 10 patients, with infections and infestations, like sepsis, being the most frequent.
No patients passed away within 30 days following their final dose of the study drug. However, one patient died 95 days later due to a cancer relapse, and another died 211 days later from pneumonia, both deaths unrelated to the study drug.
Aligning with results from Cohort 1 and earlier trials of SYN-004 in healthy individuals, none of the blood samples from patients contained detectable levels of SYN-004 at any time point.
The pharmacokinetic profile of piperacillin, which SYN-004 can metabolize, matched expectations for this patient group.
After reviewing the safety and pharmacokinetic data, the DSMC advised continuing to Cohort 3 enrollment, where the study drug (SYN-004 or placebo) will be paired with the IV beta-lactam antibiotic, cefepime.
Steven A. Shallcross, CEO of Theriva Biologics, noted, “These promising findings support the further development of SYN-004 and contribute to the growing body of evidence highlighting its therapeutic potential. Results from the first two cohorts showed that active SYN-004 is not present in the blood of allo-HCT patients following repeated oral dosing, which helps ease concerns over its absorption in patients with compromised intestinal barriers and possible interference with IV antibiotics. We greatly appreciate the tremendous support from Dr. Dubberke and his team at Washington University as we seek additional funding to conduct the third cohort and strive to enhance standard therapy for these highly vulnerable patients by addressing the challenges of broad-spectrum IV beta-lactam antibiotics.”
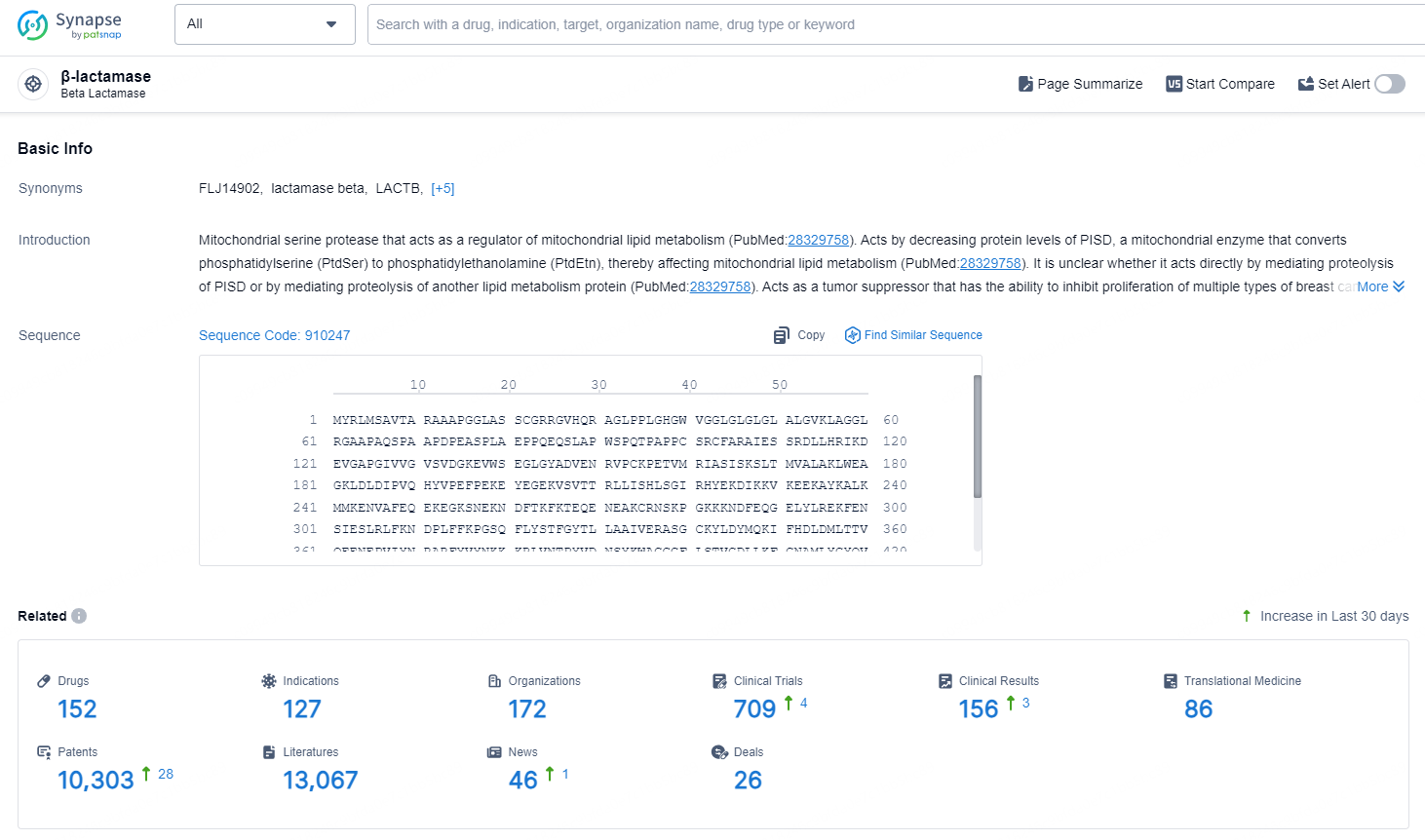
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of October 9, 2024, there are 152 investigational drug for the β-lactamase targets, including 127 indications, 172 R&D institutions involved, with related clinical trials reaching 709, and as many as 10303 patents.
Ribaxamase is an enzyme drug that targets β-lactamase and falls within the therapeutic areas of immune system diseases. Its active indication is for the prevention of bone marrow transplant rejection. The drug was developed by Theriva Biologics, Inc., and has reached the highest phase of global development at Phase 1/2.