Long-Term Control of Moderate-to-Severe Atopic Dermatitis with Almirall's EBGLYSS® (Lebrikizumab) Sustained Over Three Years in 80% of Patients
Over 80% of adults and teenagers with moderate-to-severe atopic dermatitis who showed a response to lebrikizumab treatment by the 16th week in the ADvocate 1 and 2 monotherapy trials and maintained their treatment regimen for up to three years exhibited prolonged skin clearance with monthly maintenance dosing. Almirall S.A. (BME: ALM) reported these new long-term findings from the ADjoin long-term extension study. These results are scheduled to be presented as a late-breaking abstract at the European Academy of Dermatology and Venereology (EADV) Congress, taking place from September 25-28 in Amsterdam, Netherlands.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
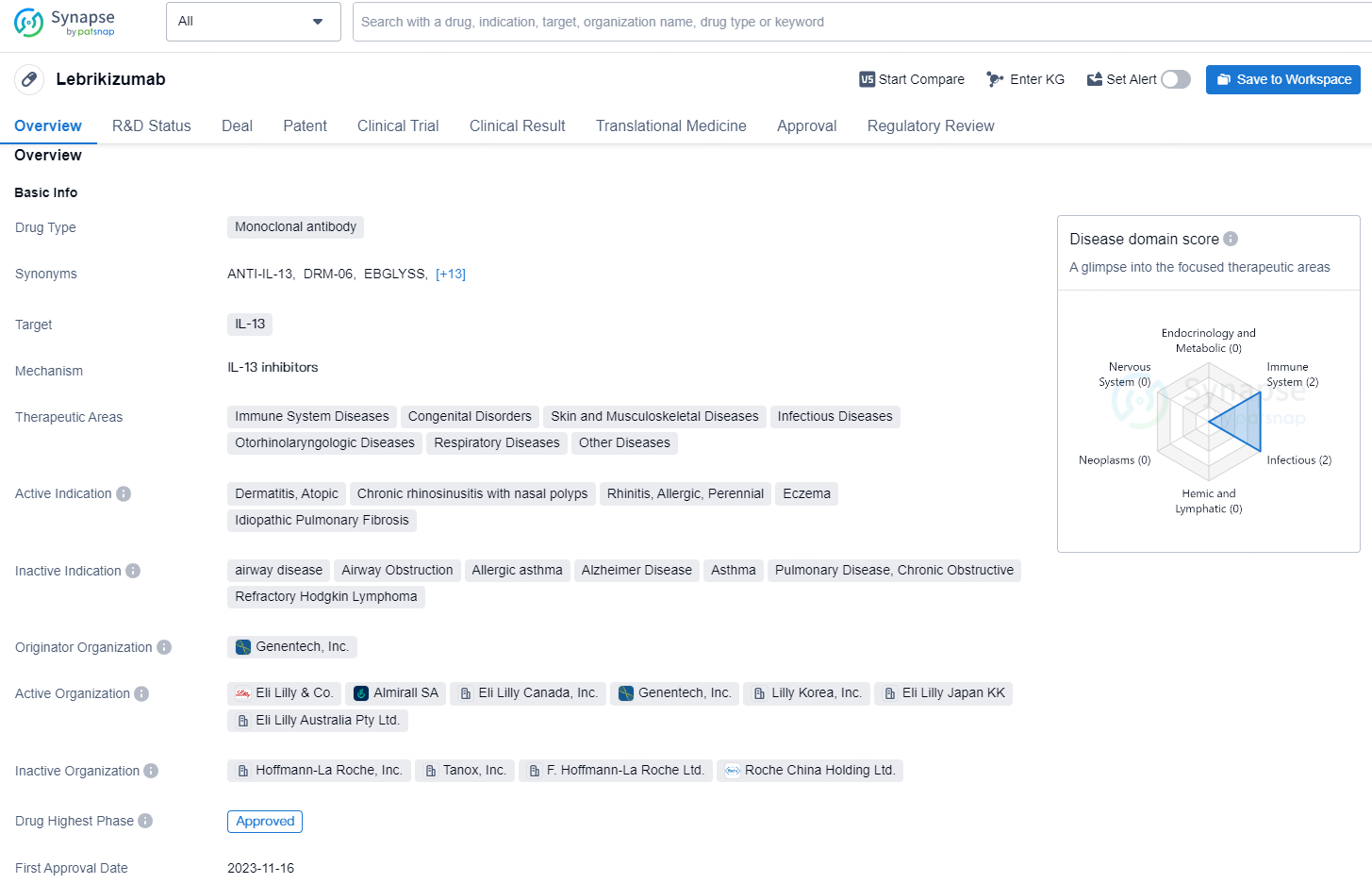
Lebrikizumab functions as an inhibitor of interleukin-13 (IL-13), effectively obstructing IL-13 signaling with a high degree of binding affinity. The cytokine IL-13 plays a pivotal role in atopic dermatitis by triggering the type-2 inflammation in the skin, resulting in skin barrier defects, pruritus, skin thickening, and infection.
"Moderate-to-severe atopic dermatitis significantly impacts patients, diminishing their quality of life and general wellbeing," stated Prof. Dr. med. Diamant Thaçi, Director at the Institute and Comprehensive Centre for Inflammation Medicine, Lübeck, Germany. "These new three-year clinical findings highlight the potential of this biologic treatment to provide sustained relief, offering long-term advantages for individuals with this chronic and recurrent condition."
Patients treated with lebrikizumab who completed 52 weeks in the ADvocate 1 or 2 trials were eligible to join the ADjoin study for an additional 100 weeks of extended therapy, totaling up to 152 weeks of continuous treatment. In the long-term extension study, participants received either 250 mg of lebrikizumab bi-weekly (Q2W) or monthly (Q4W). The sanctioned maintenance dose of lebrikizumab is 250 mg monthly (Q4W). The data provided stem from ADjoin, the extension study of the lebrikizumab trials, and covers participants who responded to lebrikizumab by Week 16 from the ADvocate 1 and ADvocate 2 studies.
At three years, 84 percent of patients receiving lebrikizumab monthly and 83 percent receiving it bi-weekly retained clear or nearly clear skin (IGA 0,1).
At three years, 87 percent of patients on a monthly regimen and 79 percent on a bi-weekly regimen achieved or maintained a minimum of 90 percent improvement in disease extent and severity (EASI-90).
At three years, 83 percent of patients treated monthly and 91 percent treated bi-weekly did not require high-potency topical corticosteroids or systemic treatments.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
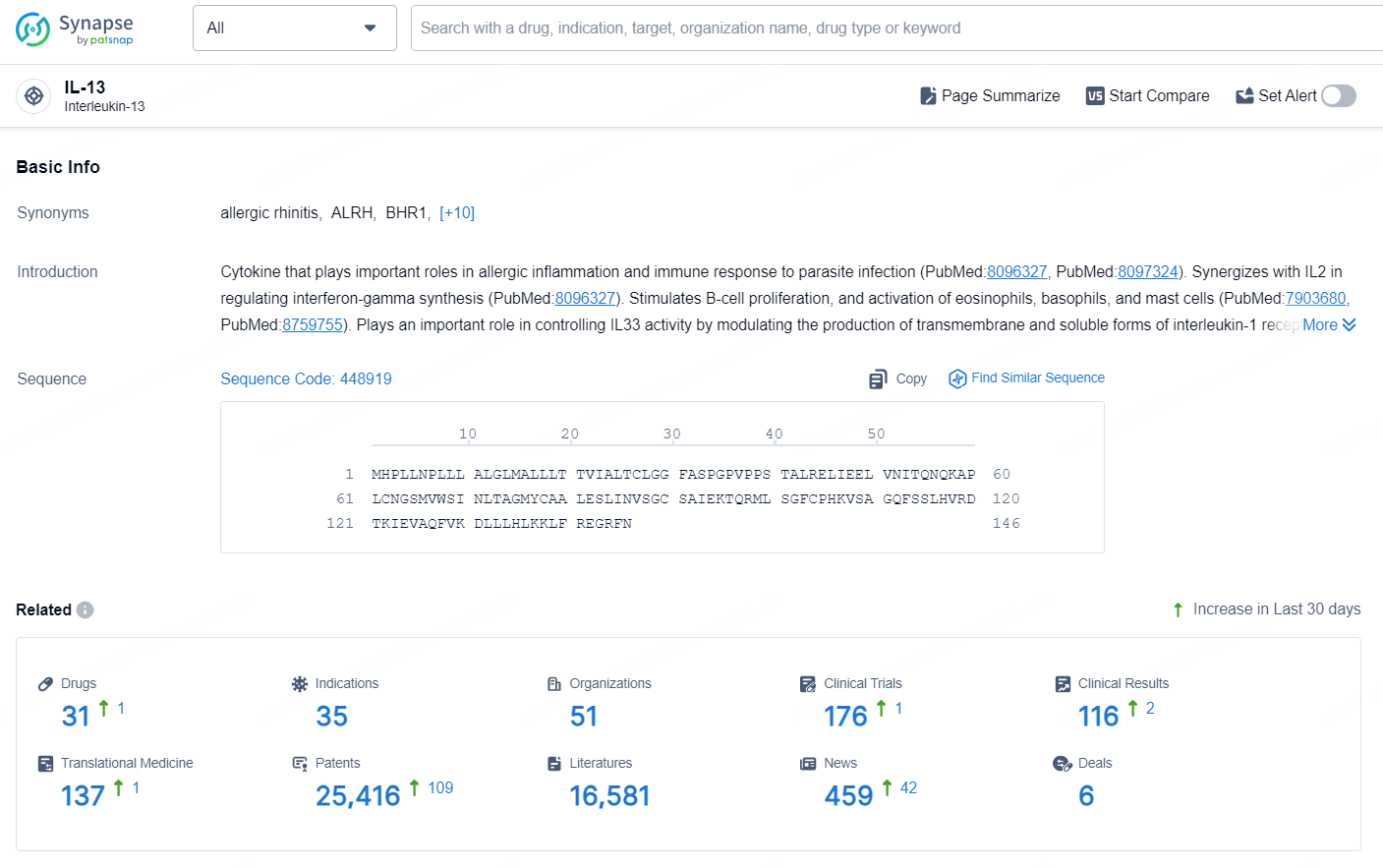
According to the data provided by the Synapse Database, As of September 24, 2024, there are 31 investigational drug for the IL-13 targets, including 35 indications, 51 R&D institutions involved, with related clinical trials reaching 176, and as many as 25416 patents.
Lebrikizumab is a monoclonal antibody drug that targets IL-13 and has been developed for the treatment of a range of therapeutic areas, including immune system diseases, congenital disorders, skin and musculoskeletal diseases, infectious diseases, otorhinolaryngologic diseases, respiratory diseases, and other diseases. The active indications for Lebrikizumab include atopic dermatitis, chronic rhinosinusitis with nasal polyps, allergic rhinitis, perennial eczema, and idiopathic pulmonary fibrosis.