Pharmaceutical Insights: tegafur's R&D Progress and its Mechanism of Action on Drug Target
Tegafur's R&D Progress
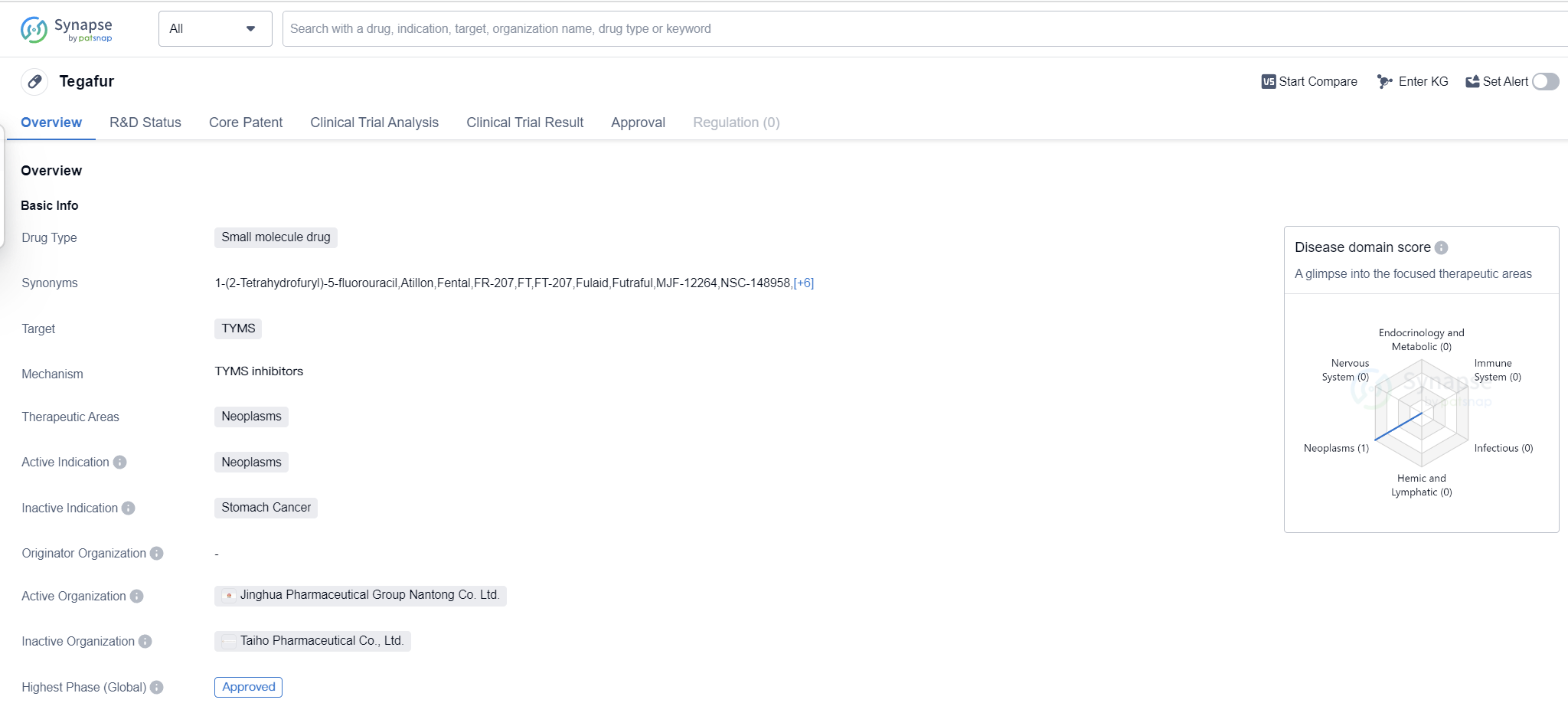
Tegafur is a small molecule drug that falls under the therapeutic area of neoplasms, which refers to abnormal growth of cells that can lead to the formation of tumors. The drug specifically targets TYMS, which stands for thymidylate synthase, an enzyme involved in DNA synthesis. Tegafur has been approved for use in the treatment of neoplasms globally.
The drug received its first approval in China in January 1983, making it one of the early drugs in the field of biomedicine. Since then, it has gained global recognition and approval. Tegafur is primarily used in the treatment of neoplasms, indicating its effectiveness in combating abnormal cell growth and potentially inhibiting tumor formation.
As a small molecule drug, Tegafur is designed to interact with specific molecular targets in the body, in this case, TYMS. By targeting this enzyme, the drug aims to disrupt the DNA synthesis process, which is crucial for the growth and proliferation of cancer cells. This mechanism of action makes Tegafur a potentially valuable tool in the fight against neoplasms.
The approval of Tegafur in globally highlights its efficacy and safety profile. The highest R&D phase of this drug is approved. This suggests that Tegafur is proven effective in treating neoplasms and meets regulatory requirements.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for tegafur: TYMS inhibitors
TYMS inhibitors are a type of medication that specifically target and inhibit the activity of the enzyme thymidylate synthase (TYMS). Thymidylate synthase is an essential enzyme involved in DNA synthesis, specifically in the conversion of deoxyuridine monophosphate (dUMP) to deoxythymidine monophosphate (dTMP). By inhibiting TYMS, these inhibitors disrupt the production of dTMP, which is necessary for DNA replication and cell division.
From a biomedical perspective, TYMS inhibitors are used in the treatment of various types of cancer, as cancer cells often have increased DNA replication and cell division rates compared to normal cells. By inhibiting TYMS, these inhibitors can slow down or halt the growth of cancer cells, ultimately leading to their death.
It is important to note that TYMS inhibitors are a specific class of drugs and not a single drug itself. Examples of TYMS inhibitors include fluorouracil (5-FU), raltitrexed, and pemetrexed. These inhibitors are typically administered through intravenous infusion or oral tablets, depending on the specific drug and the type of cancer being treated.
Overall, TYMS inhibitors play a crucial role in cancer treatment by targeting the enzyme involved in DNA synthesis and cell division, ultimately inhibiting the growth of cancer cells.
Drug Target R&D Trends for tegafur
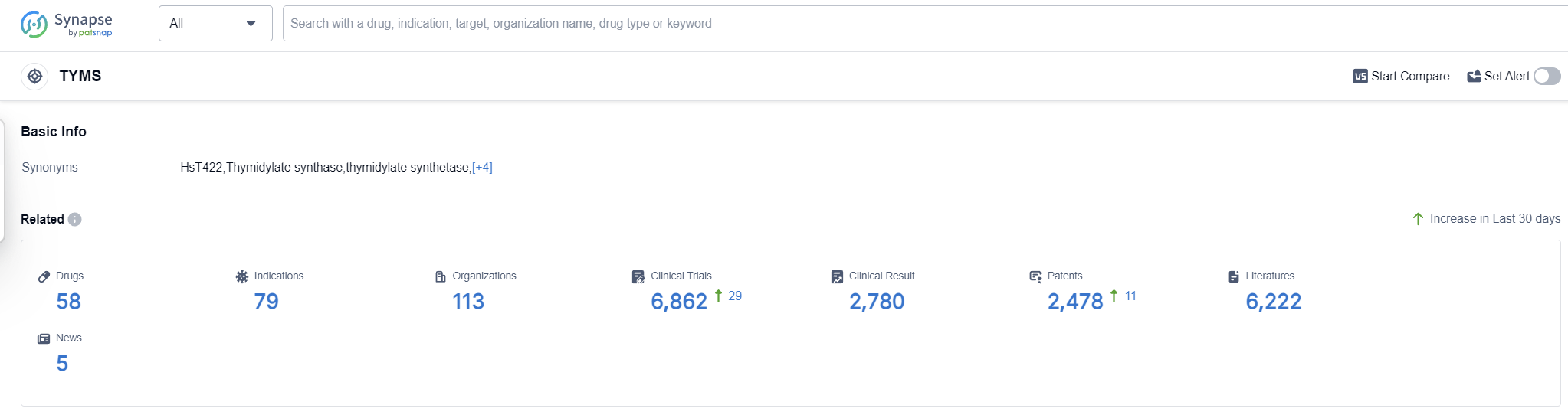
TYMS, or thymidylate synthase, plays a crucial role in the human body as an enzyme involved in DNA synthesis. It catalyzes the conversion of deoxyuridine monophosphate (dUMP) to deoxythymidine monophosphate (dTMP), a key step in the production of thymidine, an essential component of DNA. By regulating the availability of dTMP, TYMS ensures the accurate replication and repair of DNA, which is vital for cell division and growth. Additionally, TYMS is a target for certain chemotherapeutic agents that inhibit its activity, thereby disrupting DNA synthesis and inhibiting the proliferation of cancer cells. Understanding TYMS function is crucial for developing novel therapies and improving patient outcomes.
According to Patsnap Synapse, as of 13 Sep 2023, there are a total of 58 TYMS drugs worldwide, from 113 organizations, covering 79 indications, and conducting 6862 clinical trials.
Based on the analysis of the data, Otsuka Holdings Co., Ltd. is the company growing the fastest under the target TYMS, with a diverse portfolio of drugs in different stages of development. Colorectal cancer is the most common approved indication for drugs under this target. Small molecule drugs are progressing rapidly, indicating intense competition in the pharmaceutical industry. China and Japan are the leading countries in terms of development under the target TYMS. The current competitive landscape suggests a focus on developing drugs for colorectal cancer and utilizing small molecule drug technology. Future development in the target TYMS should consider expanding indications, exploring other drug types, and further collaboration with companies in China and Japan.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Tegafur is a small molecule drug that targets TYMS and is approved for the treatment of neoplasms. Its first approval in China in 1983 paved the way for its global recognition. With its mechanism of action disrupting DNA synthesis, Tegafur holds promise in combating abnormal cell growth and inhibiting tumor formation. The highest R&D phase of this drug is approved.