Understanding the Development Progress of JAK2 Inhibitor
JAK2 (Janus kinase 2) is an enzyme that plays a crucial role in the human body, particularly in the regulation of cell growth and differentiation. It is a member of the JAK family of kinases and is primarily involved in the signaling pathway of cytokines and growth factors. JAK2 is responsible for transmitting signals from these molecules to the nucleus of cells, ultimately leading to the activation of various genes involved in cell proliferation and differentiation. Dysregulation or mutations in JAK2 can lead to the development of certain diseases, including myeloproliferative neoplasms and autoimmune disorders. Understanding the role of JAK2 is essential for the development of targeted therapies in the pharmaceutical industry.
JAK2 Competitive Landscape
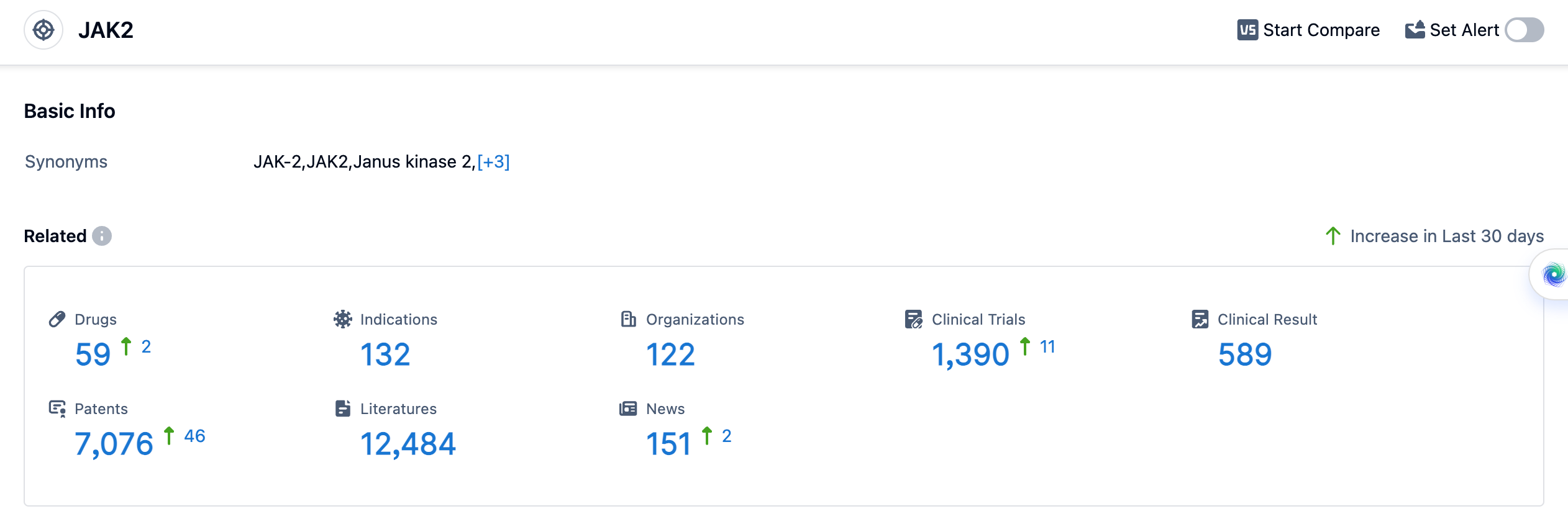
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 11 Sep 2023, there are a total of 59 JAK2 drugs worldwide, from 122 organizations, covering 132 indications, and conducting 1390 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
The analysis of target JAK2 reveals a competitive landscape with multiple companies actively involved in the development of drugs. Novartis AG has the highest stage of development, followed by other pharmaceutical companies such as Eli Lilly & Co., Incyte Corp., AbbVie, Inc., Pfizer Inc., Bristol Myers Squibb Co., and more. The drug types progressing most rapidly under the current target JAK2 are small molecule drugs, chemical drugs, PROTACs, and unknown drug types. The indications for the drugs under target JAK2 cover a wide range of diseases, including Rheumatoid Arthritis, Primary Myelofibrosis, Dermatitis, Atopic, and more. Small molecule drugs are progressing rapidly, indicating intense competition in the market. The United States, European Union, Japan, and China are among the countries/locations with significant progress in the development of drugs under target JAK2. China, in particular, has shown progress with approved and preclinical drugs. Overall, the target JAK2 presents a competitive landscape with potential for future development and innovation in the pharmaceutical industry.
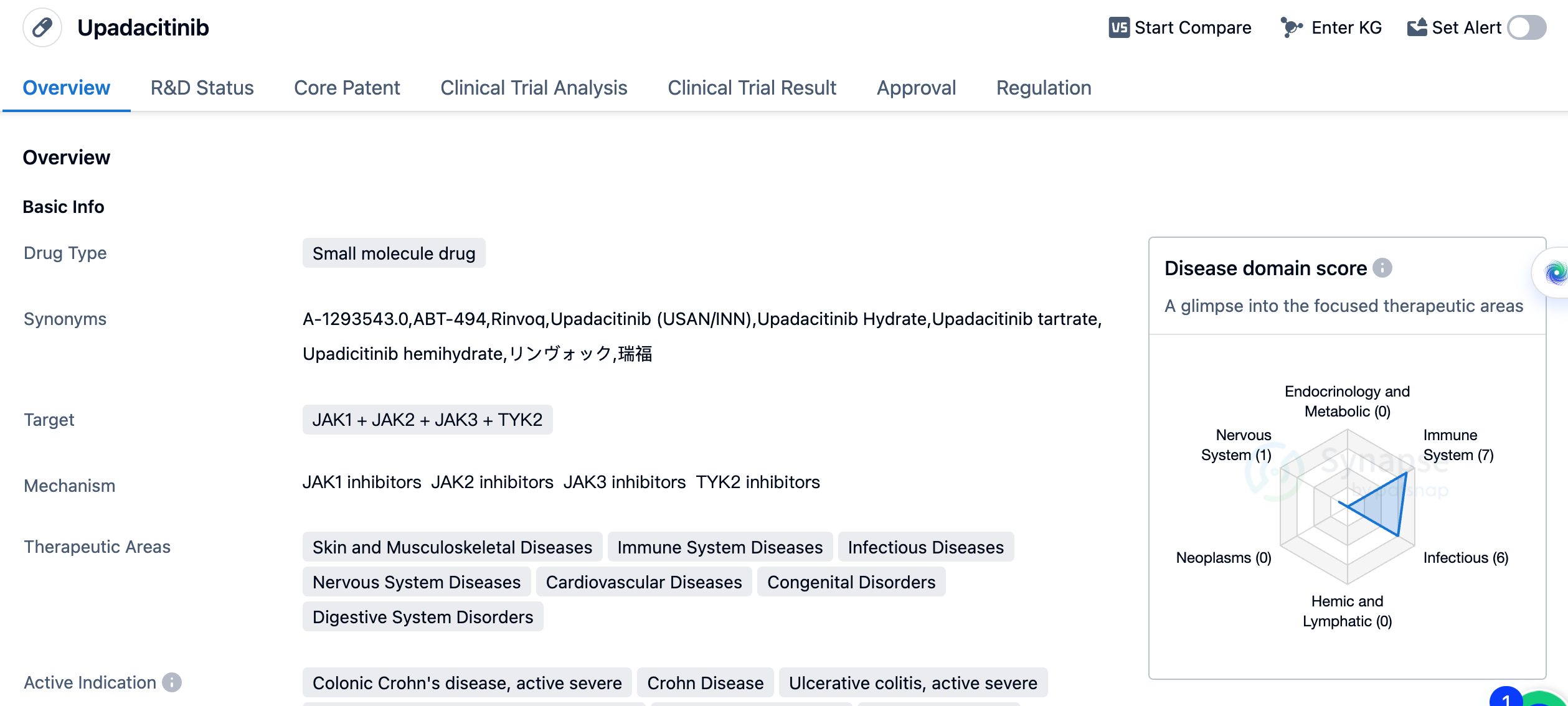
Key drug:Upadacitinib
Upadacitinib is a small molecule drug that targets JAK1, JAK2, JAK3, and TYK2. It has been developed by AbbVie, Inc. and has received approval for use in various therapeutic areas. These include skin and musculoskeletal diseases, immune system diseases, infectious diseases, nervous system diseases, cardiovascular diseases, congenital disorders, and digestive system disorders.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The drug received its first approval in the United States in August 2019 and has also been approved in China. It has undergone priority review, indicating its potential to address unmet medical needs. Additionally, it has been designated as an orphan drug, suggesting its use in treating rare diseases. The drug has also been part of a special review project, which further highlights its significance in the pharmaceutical industry.
Upadacitinib's approval in multiple therapeutic areas and its targeting of various JAK proteins make it a promising drug in the field of biomedicine. Its ability to treat a wide range of diseases, including severe conditions like colonic Crohn's disease and systemic lupus erythematosus, demonstrates its potential to improve patient outcomes and quality of life. In summary, Upadacitinib is a small molecule drug developed by AbbVie, Inc. that targets JAK1, JAK2, JAK3, and TYK2. It has received approval for use in several therapeutic areas and has shown efficacy in treating various indications. Its approval in the United States and China, as well as its designation as an orphan drug and involvement in a special review project, further emphasize its importance in the pharmaceutical industry.
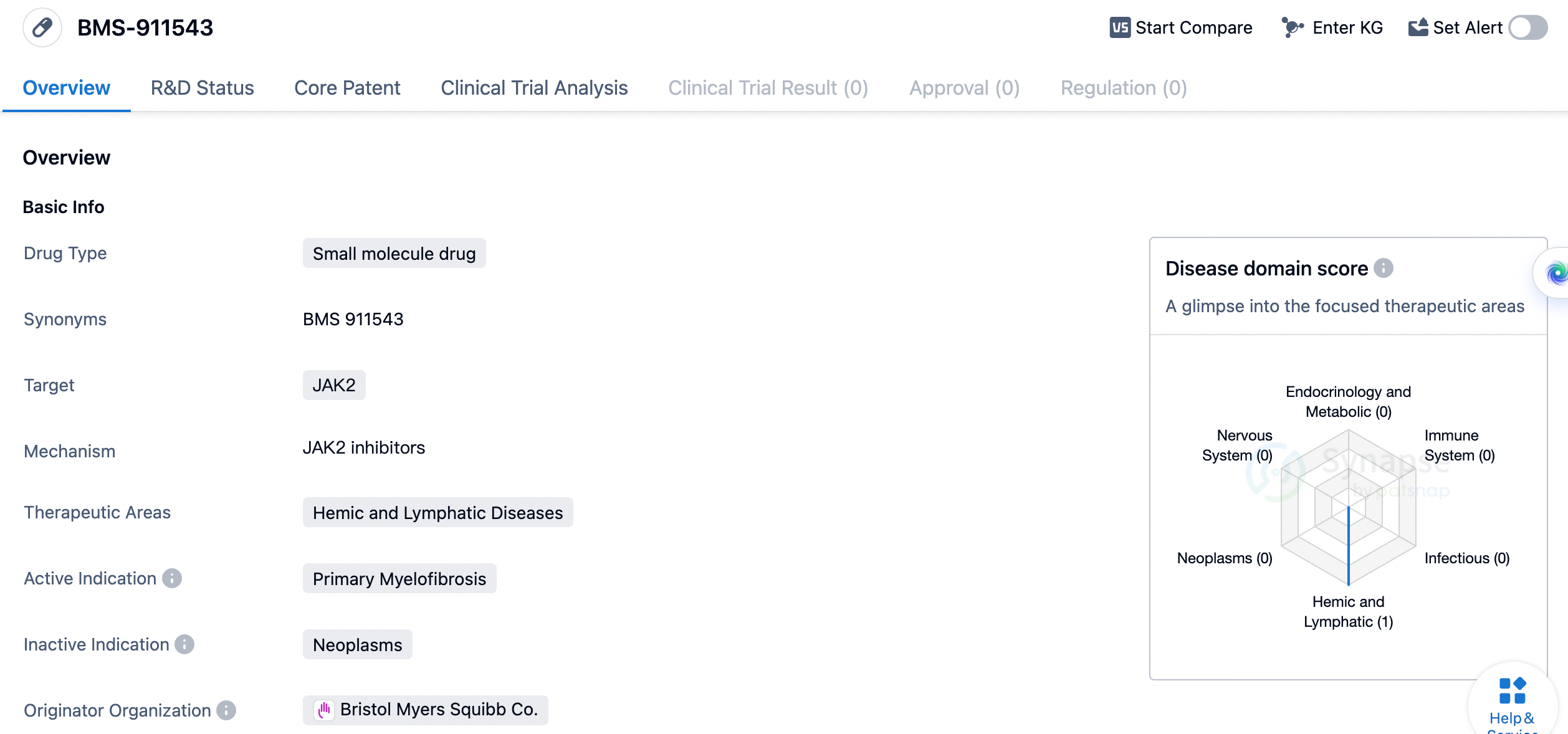
new drugs:BMS-911543
BMS-911543 is a small molecule drug developed by Bristol Myers Squibb Co. It falls under the therapeutic area of Hemic and Lymphatic Diseases and specifically targets JAK2, a protein associated with various blood disorders. The drug is currently in Phase 2, which indicates that it has already undergone initial testing for safety and efficacy in a small group of patients. The primary indication for BMS-911543 is Primary Myelofibrosis, a rare and chronic blood cancer characterized by the abnormal production and accumulation of fibrous tissue in the bone marrow. This condition can lead to anemia, enlarged spleen, and other complications. By targeting JAK2, BMS-911543 aims to inhibit the activity of this protein, which is known to play a crucial role in the development and progression of myelofibrosis.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
As a small molecule drug, BMS-911543 is designed to be orally administered, allowing for convenient and non-invasive treatment. Small molecule drugs are typically synthesized chemically and have a relatively low molecular weight, enabling them to penetrate cells and interact with specific targets within the body. Overall, BMS-911543 shows promise as a potential treatment for Primary Myelofibrosis, a challenging and debilitating blood disorder. Its specific targeting of JAK2 and its small molecule nature make it a potentially effective and convenient therapeutic option. However, further research and clinical trials are needed to determine its safety, efficacy, and potential benefits for patients with this condition.