Unveiling the Updated Email Alert Feature in Synapse Database: A Game-changer in Data Management
The field of medicine is evolving rapidly, with the market constantly changing. Every new technology and research in the medical field has the potential to profoundly impact drug development. Through intelligence monitoring, you can stay informed about the latest industry trends and competitors' movements in real-time. This enables quick adjustments to research and development strategies, allowing you to seize opportunities. Monitoring the latest scientific research and clinical trial data helps expand research perspectives, discover new directions, and drive pharmaceutical innovation. In the process of new drug development, understanding competitors' dynamics can assist companies in timely strategic adjustments, avoiding potential regulatory risks, and increasing the likelihood of research and development success.
Currently, there is a wide variety of content in pharmaceutical intelligence, covering drug pipelines, regulatory information, clinical data, patents, literature, and news. According to statistics from the Patsnap Synapse database, there are over 60 drugs, 3000+ pharmaceutical patents, 150+ clinical trials, 5000+ pharmaceutical literature, and 500+ pharmaceutical news disclosures every day. How to accurately track relevant pharmaceutical intelligence of interest in this massive amount of data? The powerful intelligence alert function of the Patsnap Synapse database makes it easy for you to complete various monitoring tasks.
We divide new drug research and development-related events into 8 major categories, which are further subdivided into 60 subcategories. Each category of events includes the date of the event and the content of the event. You can obtain exactly these events through different search settings.
| Categories | Subcategories |
| R&D Progress |
|
| Regulation Review |
|
| Clinical Trial |
|
| Deal |
|
| Patent |
|
| Literature | - |
| News | - |
| Investment & Financing |
|
Operation Guide
Set Alert
You can set alert after conducting searches in the drug, clinical trial, clinical results, and patent sections. Additionally, alert can be set up on the drug, target, indication, drug type, institution, clinical trial, and patent details page.
1.Alert for Search Queries
Navigate to the advanced search page for drugs/clinical trials/clinical results/patents, input your search criteria, click on "Set Alert", select events, and you can start monitoring your search queries.
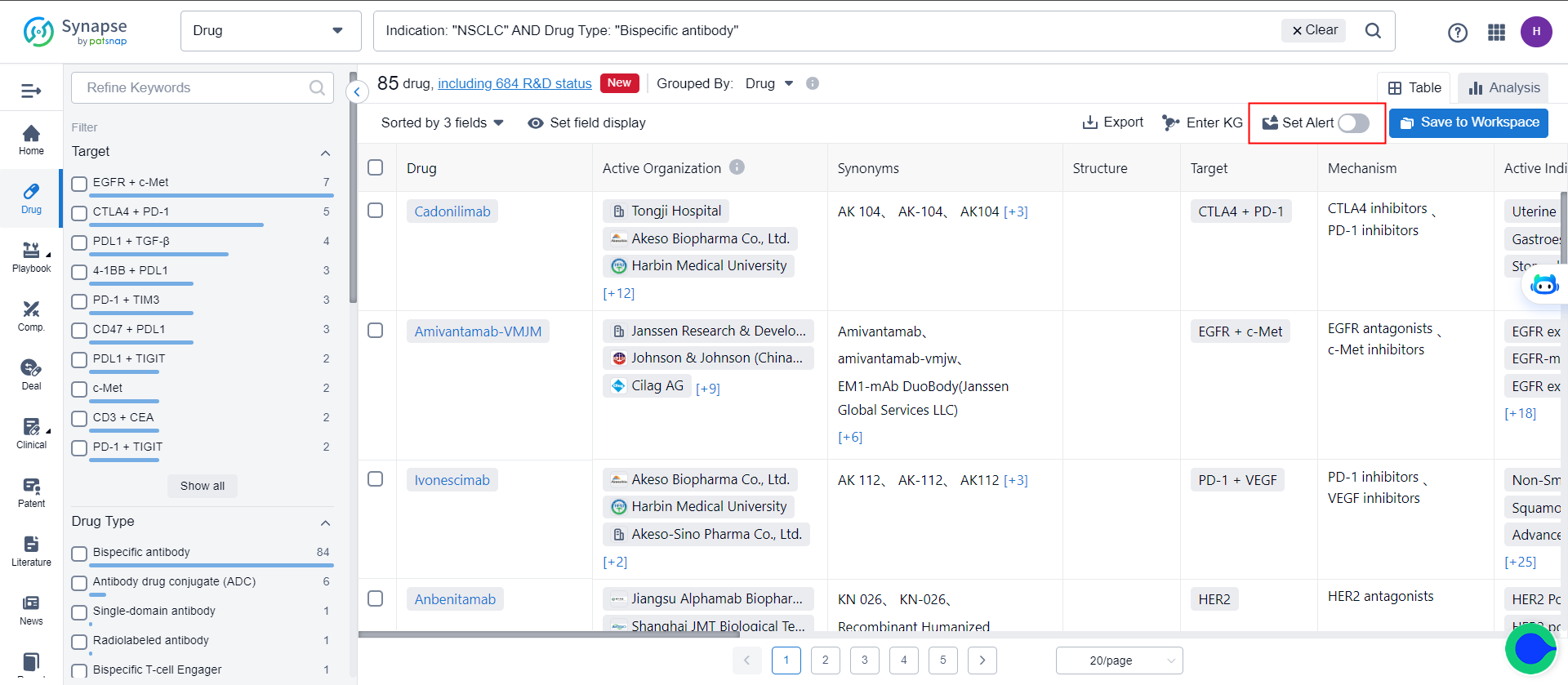
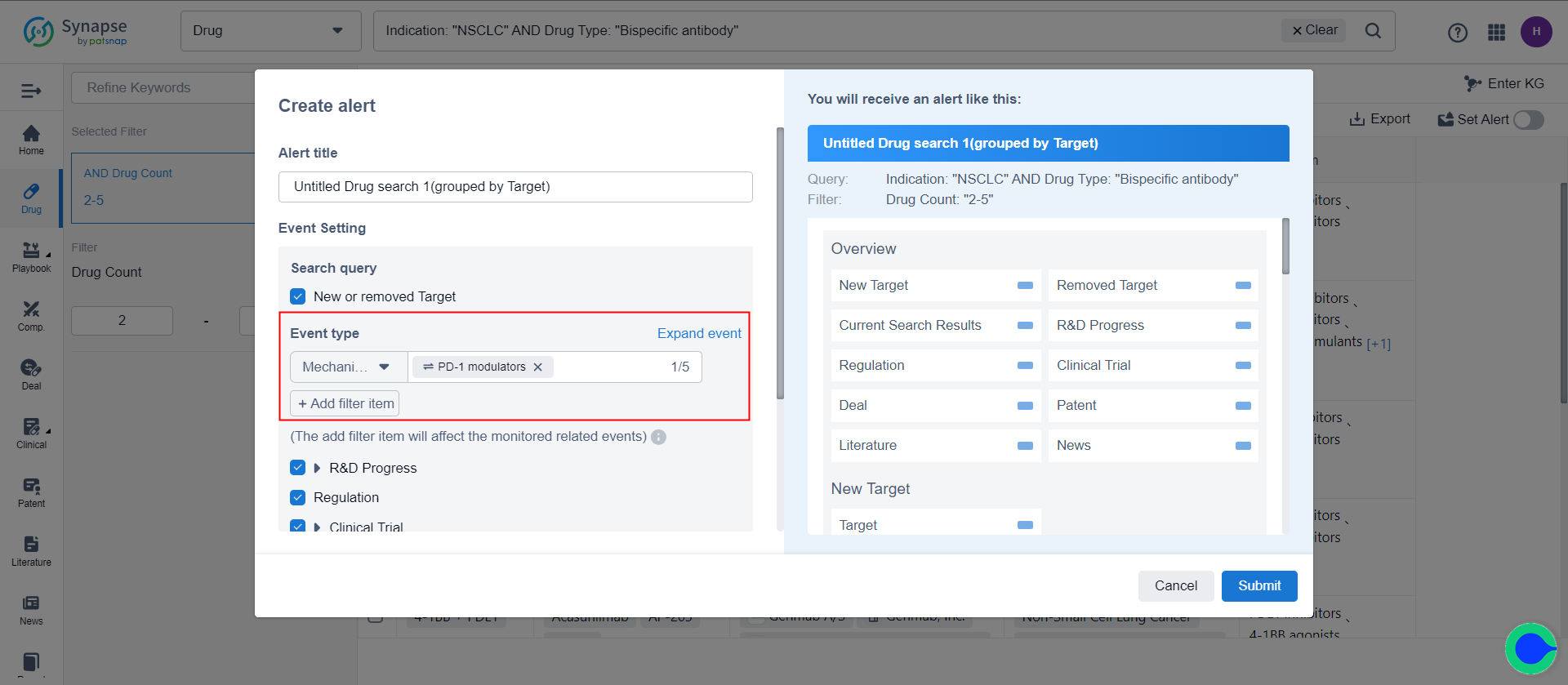
The drug search results can be adjusted based on aggregation dimensions. After adjustment, it is possible to monitor specific dimensions in the list. For example, after re-aggregating based on target, it is possible to monitor the targets in the list.
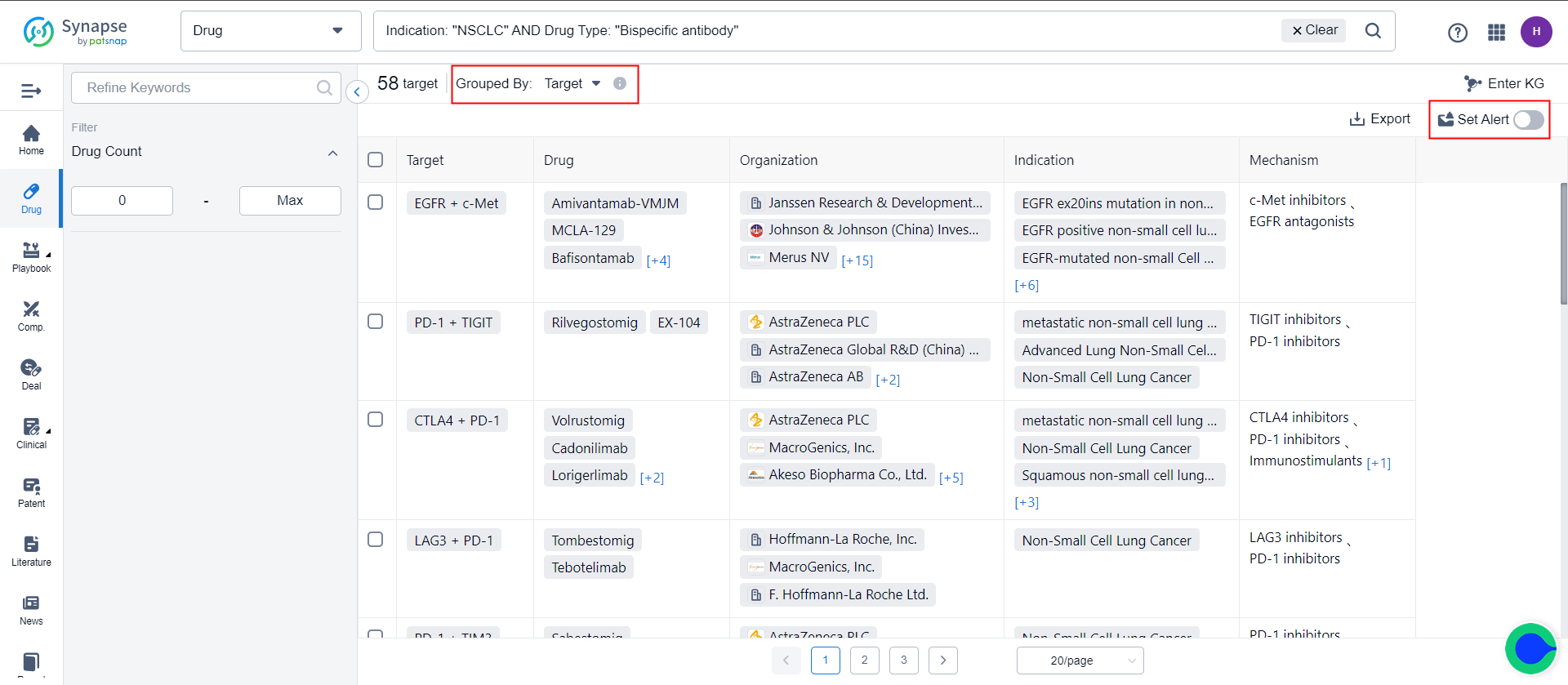
The new/removed-type events refer to the set difference between the list in the current retrieval formula and the list in the previous retrieval formula. This difference may arise due to data updates or corrections. When the number of results within the retrieval formula is less than a certain quantity, it is possible to choose more events. In addition to monitoring changes in the retrieval formula list, events related to each element in the list can also be monitored. The list of elements in the retrieval formula is updated in real-time, meaning that when new elements are added, events related to both the existing and new elements are monitored simultaneously.
Taking the example of drug search results in the target dimension, when the number of targets is less than 50, in addition to tracking the addition or deletion of targets in the retrieval formula, events related to all targets in the retrieval formula can be monitored. This includes information such as the research and development progress, clinical progress, and clinical outcomes related to these targets. Assuming that in the current retrieval formula (20 targets), there is one newly added target compared to the previous retrieval formula (21 targets), and two targets have been removed. The monitoring scope for the current formula is thus 20 targets, including one newly added target, but excluding three targets that have been removed.
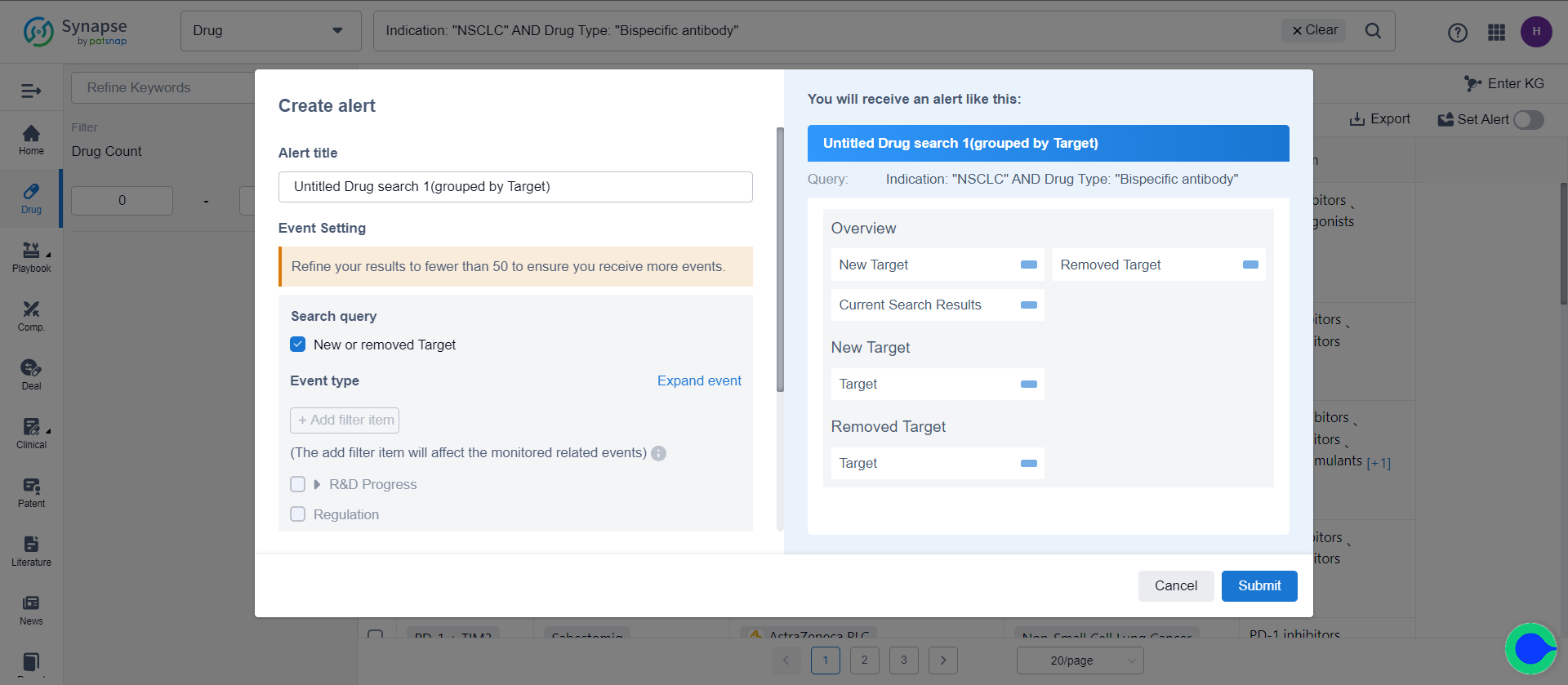
Among them, target/indication/organization/drug type support filtering events. For example, it is possible to limit the mechanism of action for targets, further narrowing down the scope of event notifications.
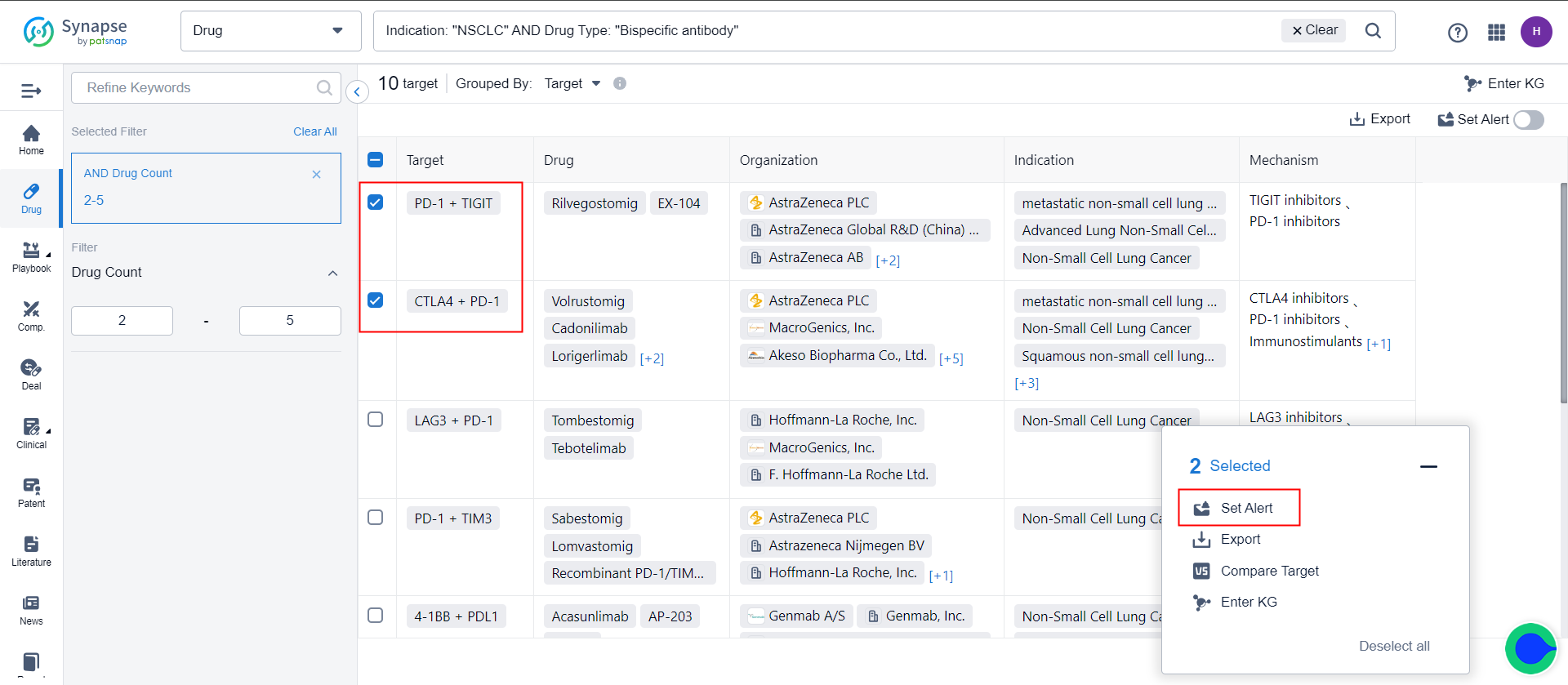
Additionally, it is possible to create monitoring in bulk by selecting multiple search results and checking them.
2.Detail Page Alerts
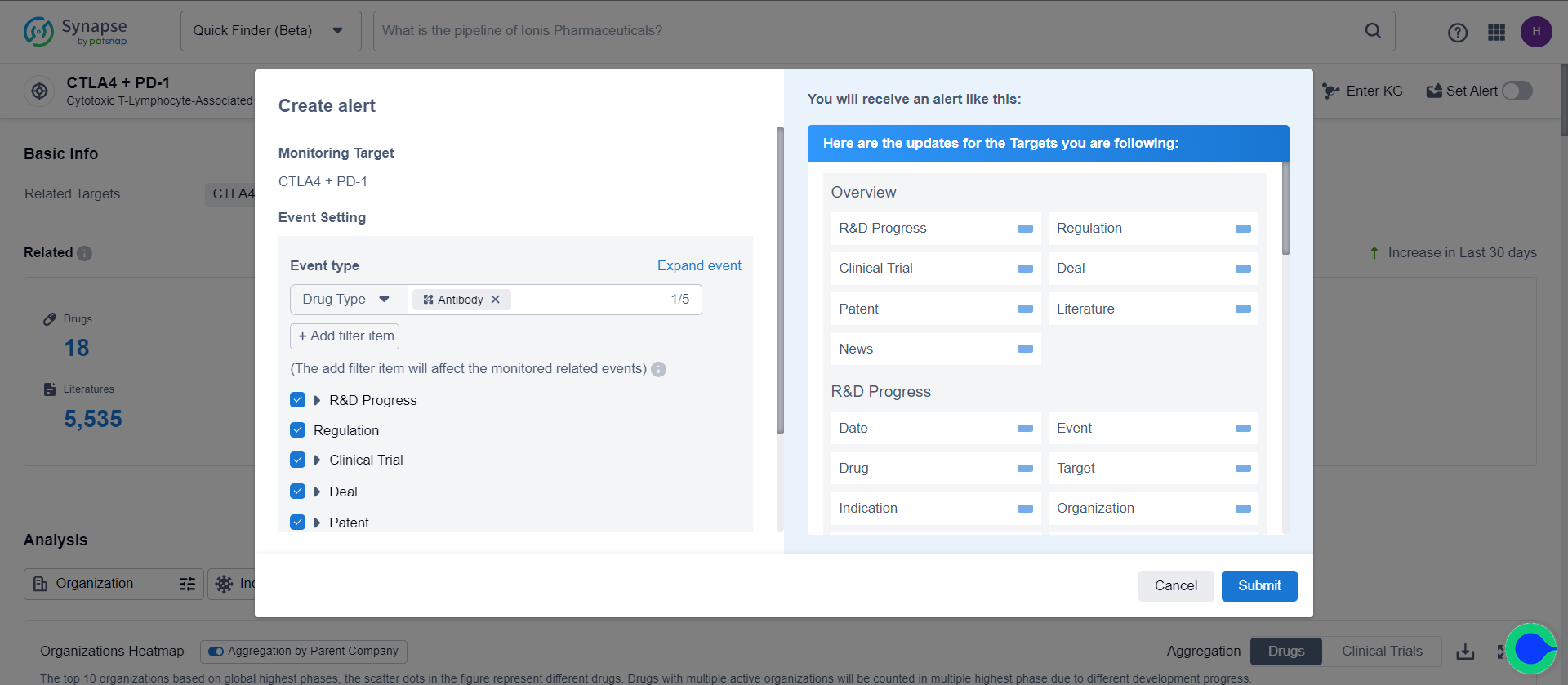
Enter the Drug/Target/Indications/Drug Type/Institution/Clinical Trial/Patent Details page, click " Set Alert ", choose events, and you can start monitoring.
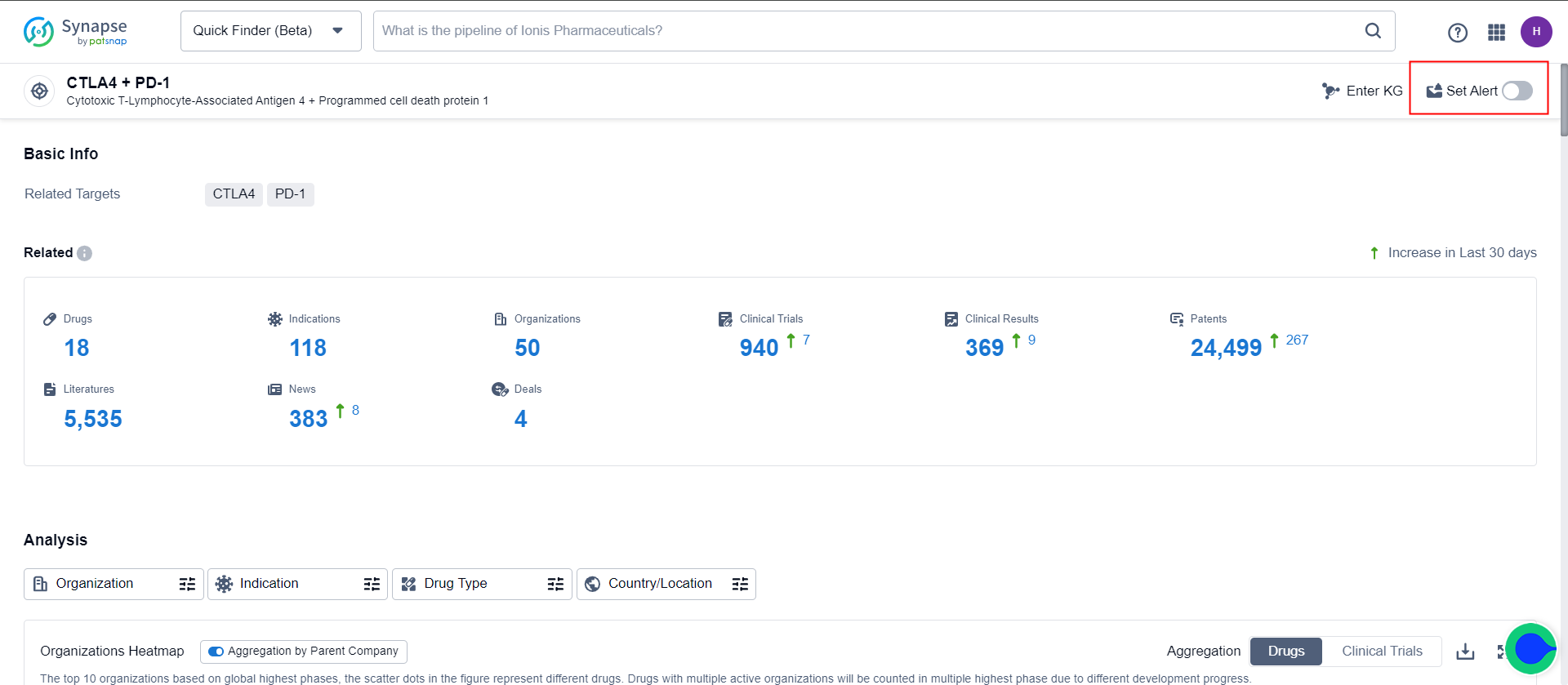
Targets/indications/organizations/drug types supports event filtering. For example, on the “CTLA4 + PD-1” details page, specifying the drug type as antibodies enables monitoring of events related to “CTLA4 + PD-1” antibodies.
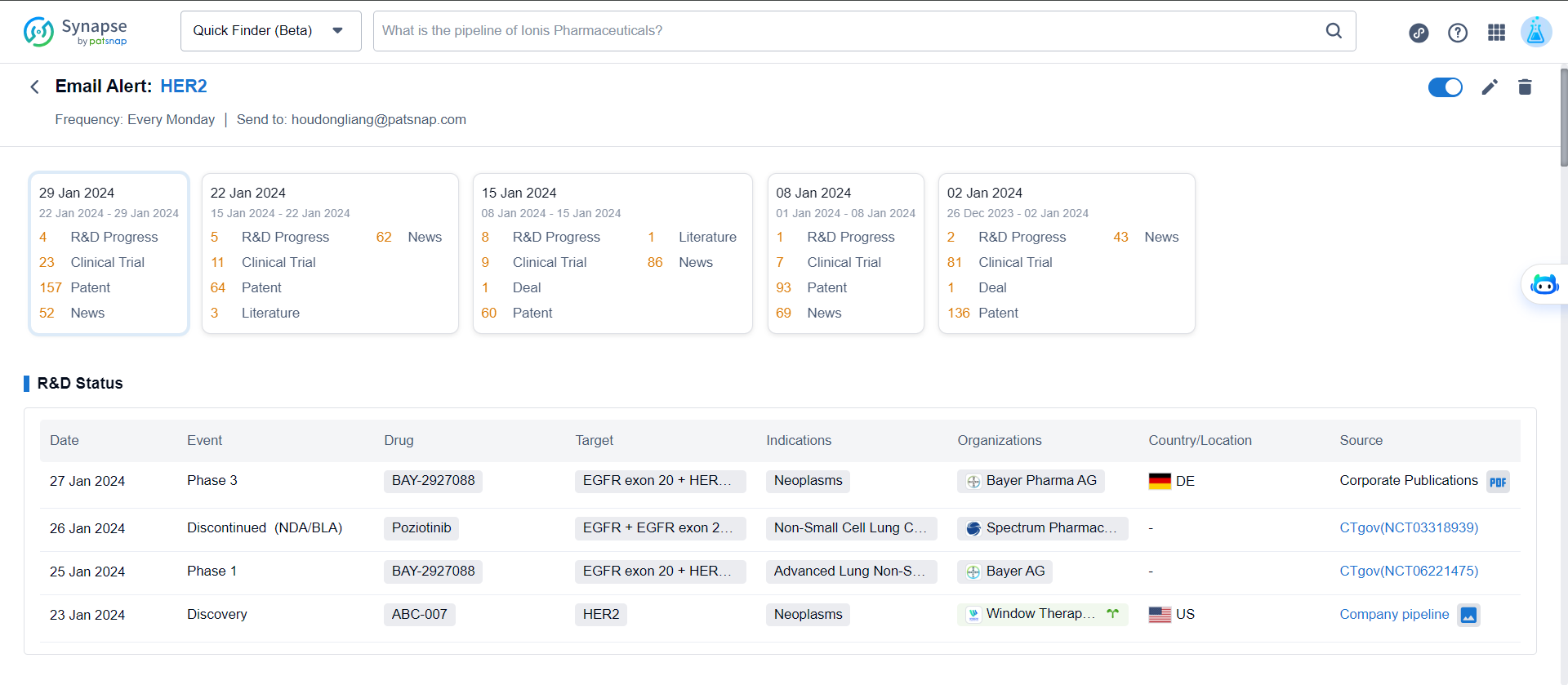
View Alert Reports
Alert reports will be sent via email. Due to limitations on email data size, the complete alert report can be viewed by logging into the web platform. The web platform retains the most recent 30 monitoring reports.
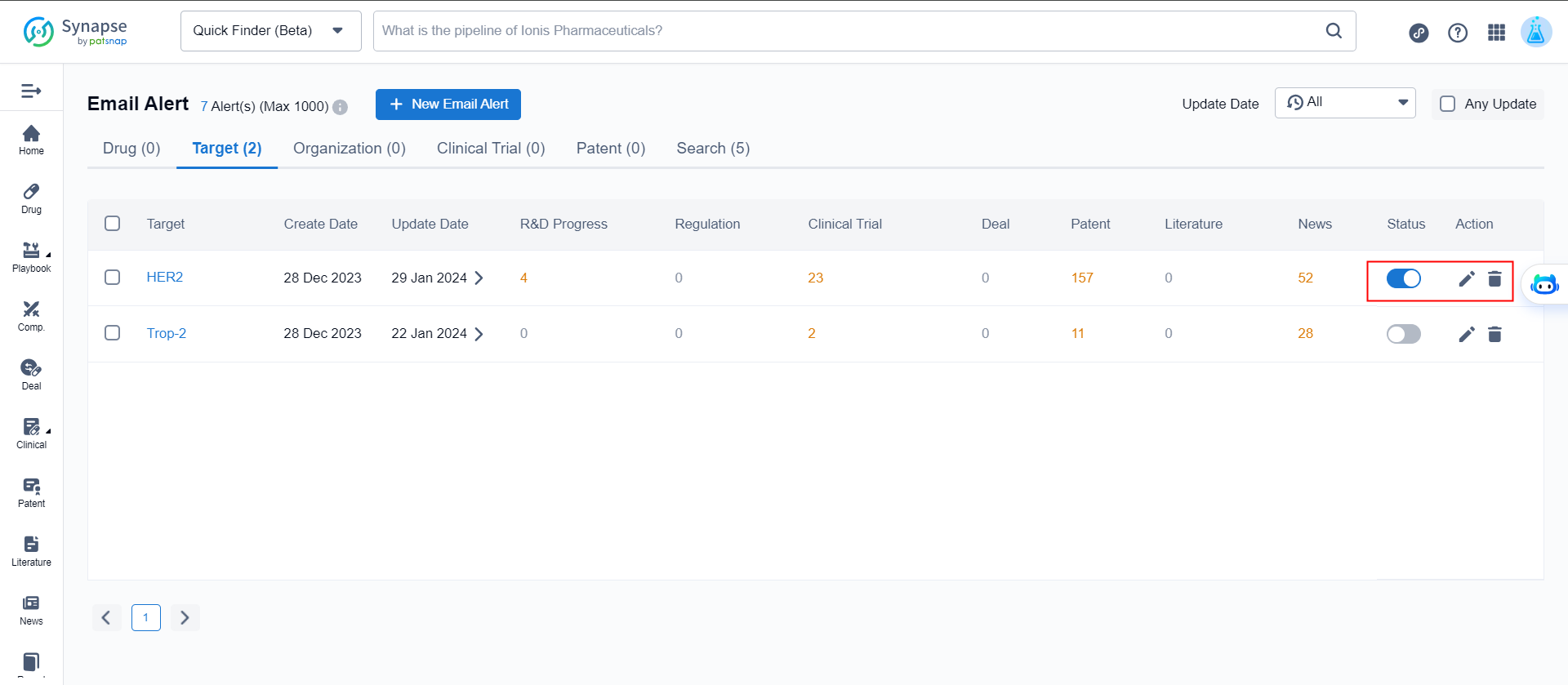
Manage Alert
On the alert management page, you can reset monitoring events, adjust the frequency of notifications, and modify the recipients. Additionally, you have the option to toggle on/off or delete alert.
Use cases
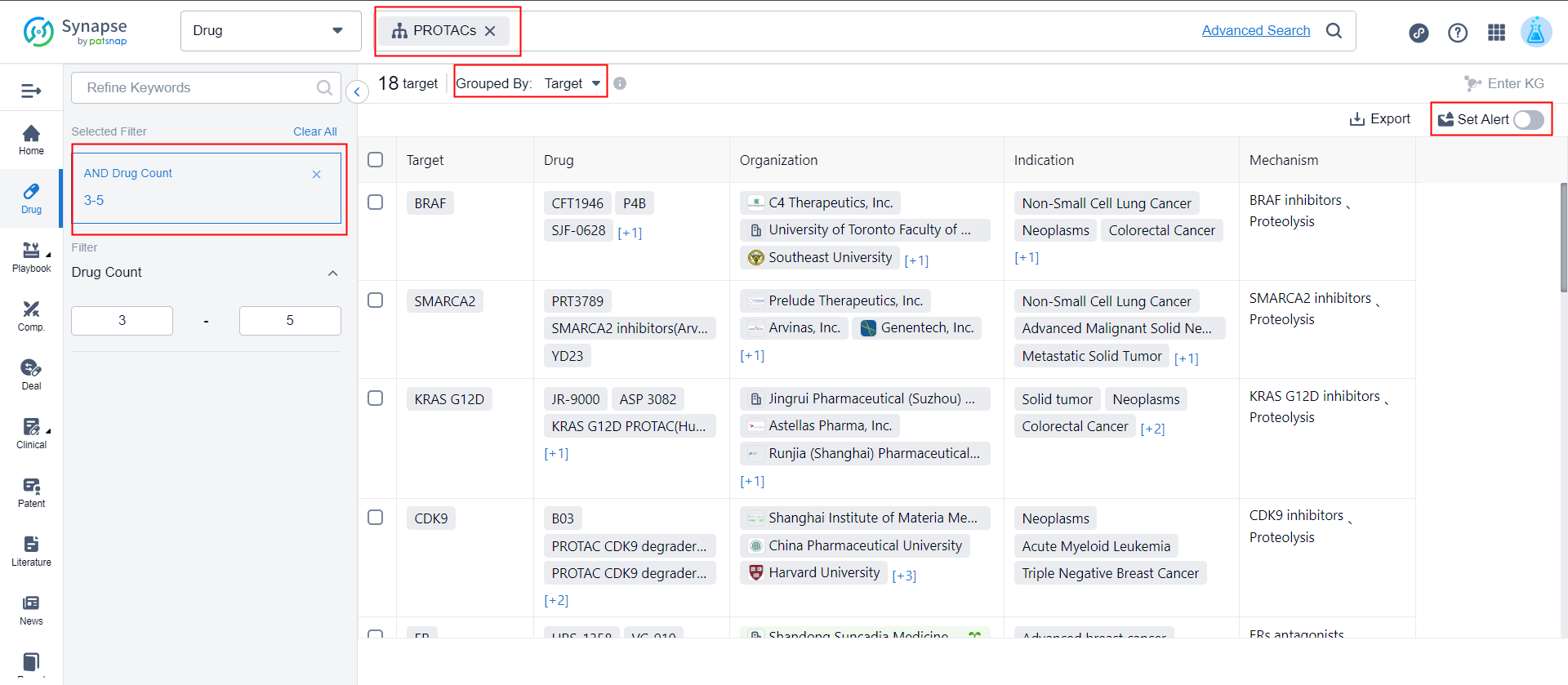
1. What are the recent potential targets for PROTAC drugs? What are the recent research and development developments on these targets?
In the advanced drug search, enter the drug type as "PROTACs", adjust the dimension as "target", filter the number of related drugs as "3-5", and click to set alert to complete.
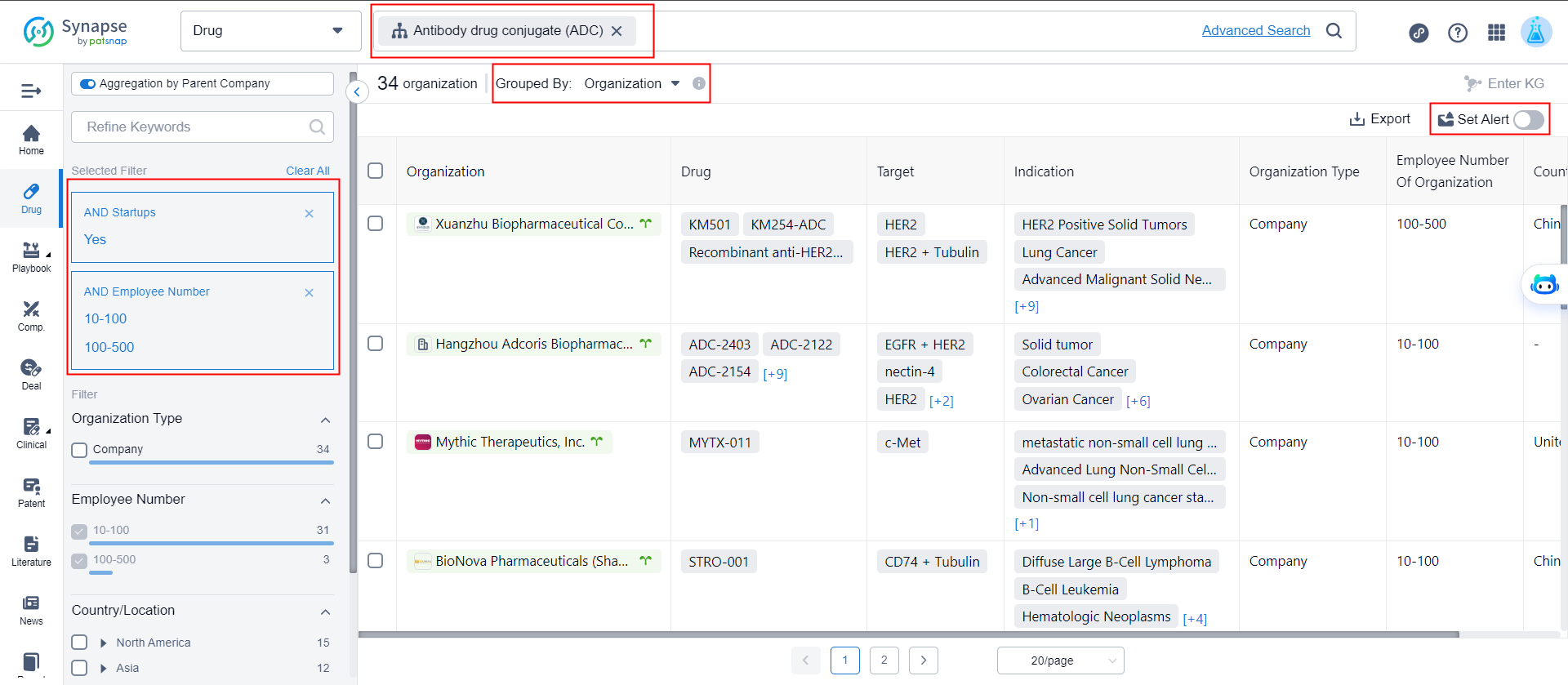
2. What are the recent startups in the ADC field? What new pipelines are coming from these startups?
In the advanced drug search, enter the drug type as "ADC", the adjustment dimension as "organization", the screening startup company as "Yes" and the number of employees as "10-500", click to set alert to complete.
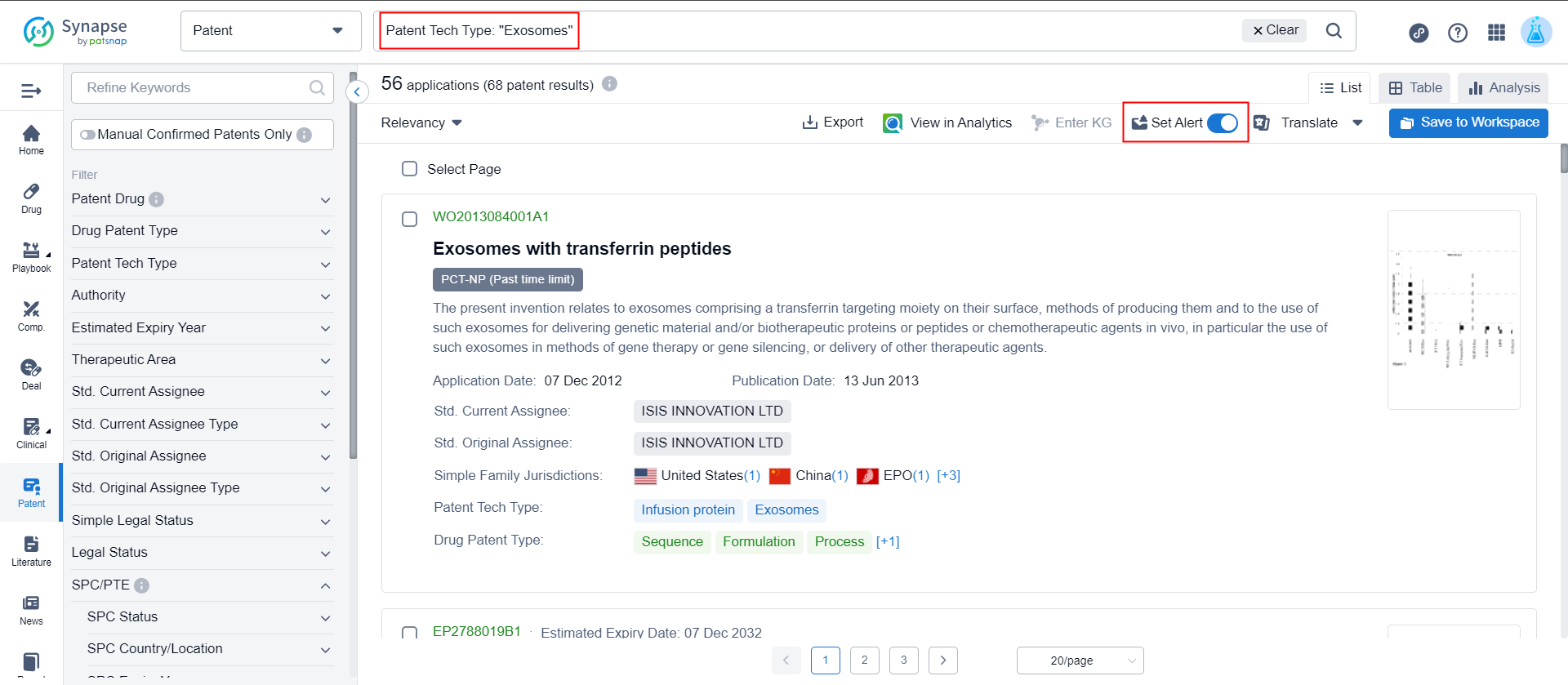
3. What are the latest published patents on exosomes?
In the patent advanced search, enter the patent technology type "exosomes" and click to set alert to complete.
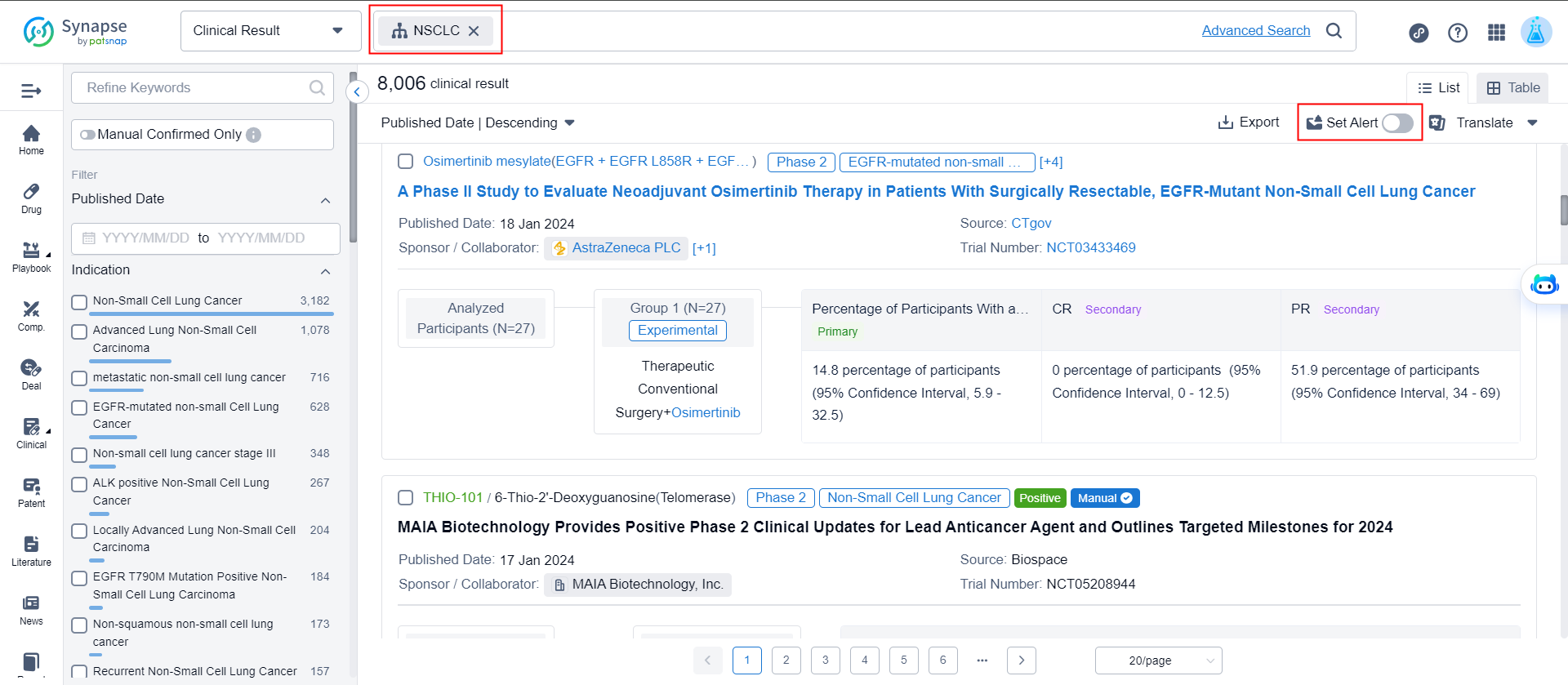
4. What are the latest clinical trial results for non-small cell lung cancer?
In the advanced search of clinical results, enter the indication as "non-small cell lung cancer" and click to set alert to complete.
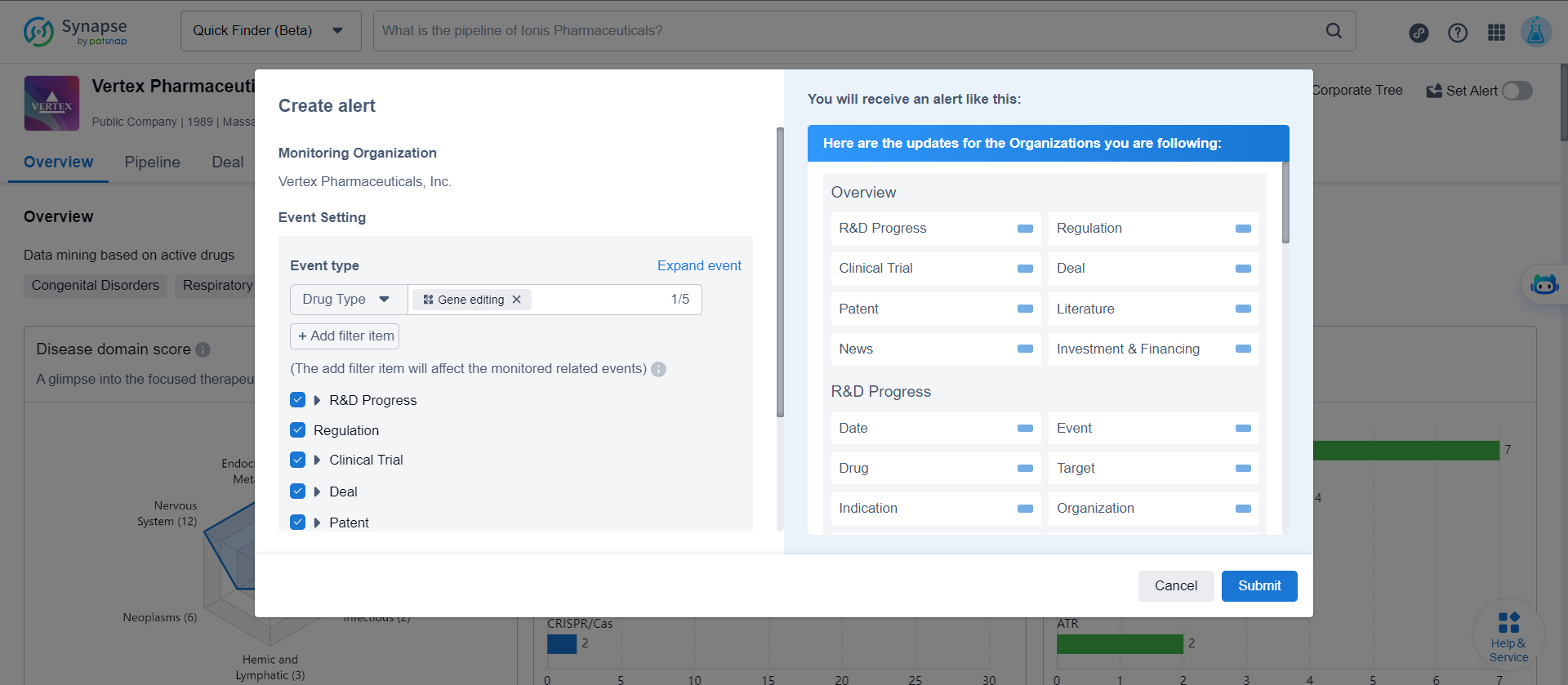
5. What are the latest research and development progress of Vertex gene editing drugs?
On the Vertex details page, click to set alert, add the filter drug type as "gene editing".