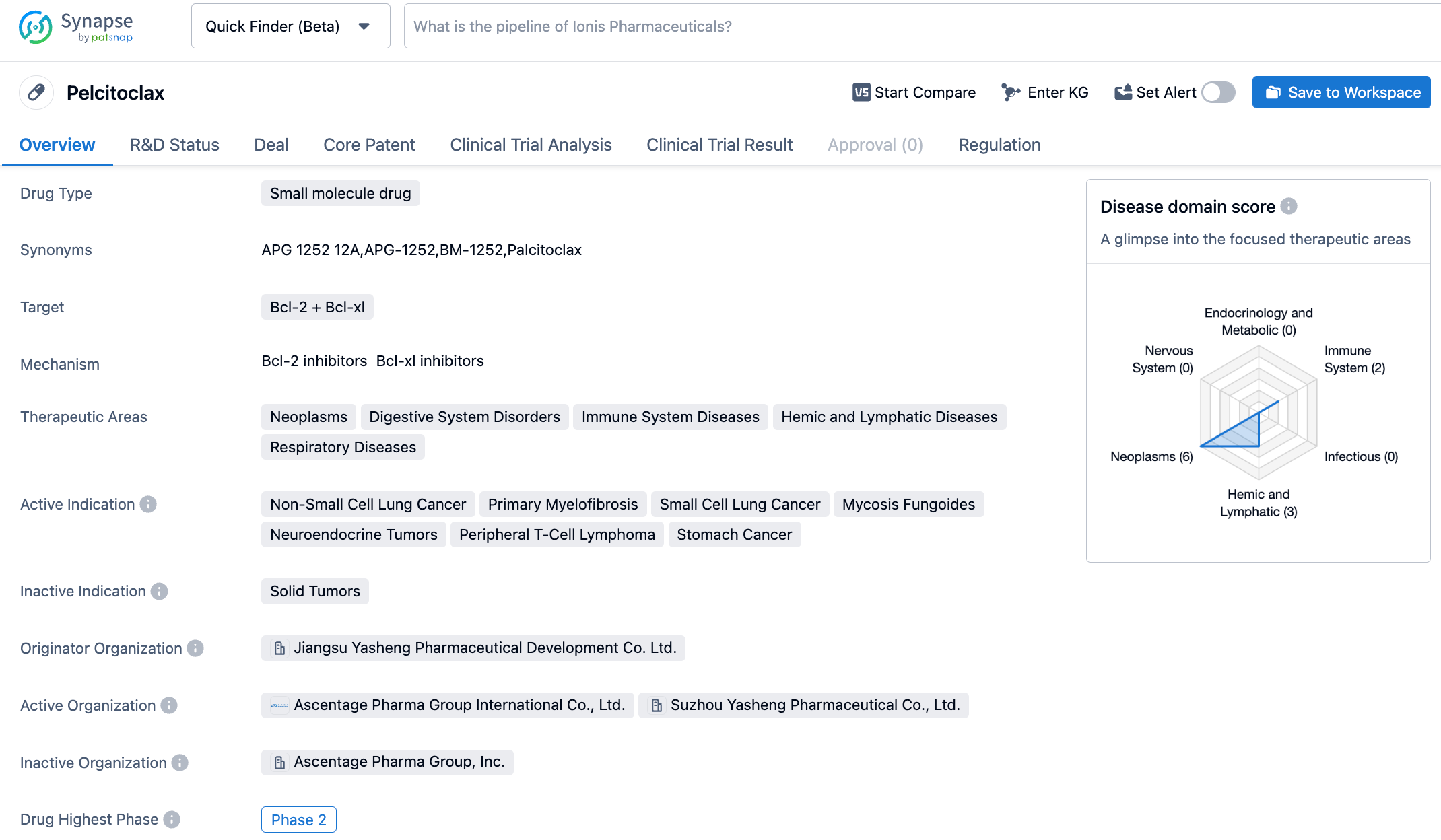
Yasheng Pharmaceutical's Bcl-2/Bcl-xL dual-target inhibitor, APG-1252, releases its latest Phase 1b clinical trial data for the treatment of non-small cell lung cancer
On October 23, 2023, Yasheng Pharmaceutical announced the latest clinical data for the Bcl-2/Bcl-xL dual target inhibitor APG-1252 in combination with Osimertinib for the treatment of non-small cell lung cancer (NSCLC) patients carrying EGFR mutations at the 2023 European Society for Medical Oncology (ESMO) annual meeting, in the form of a mini oral presentation.
APG-1252 is a novel Bcl-2/Bcl-xL dual-target inhibitor independently developed by Yasheng Pharmaceuticals with Best-in-class potential. It can achieve the effect of treating NSCLC and other solid tumors and hematologic malignancies by selectively inhibiting Bcl-2 and Bcl-xL proteins to repair cell apoptosis.
The data of APG-1252 shown at the ESMO meeting indicate that this product in combination with Osimertinib has good therapeutic potential for patients with EGFR-mutated NSCLC. This study is an open, multi-center phase Ib study conducted in China, aiming to assess the safety, tolerability, pharmacokinetics (PK), and antitumor activity of APG-1252 in combination with Osimertinib for patients with EGFR-mutated NSCLC. The results showed that among the 26 patients who had not received EGFR tyrosine kinase inhibitor (TKI) treatment, 21 achieved partial remission (PR), with an objective response rate (ORR) of 80.8%; and among the 16 patients with TP53 and EGFR co-mutations, 14 had PR, with an ORR of 87.5%.
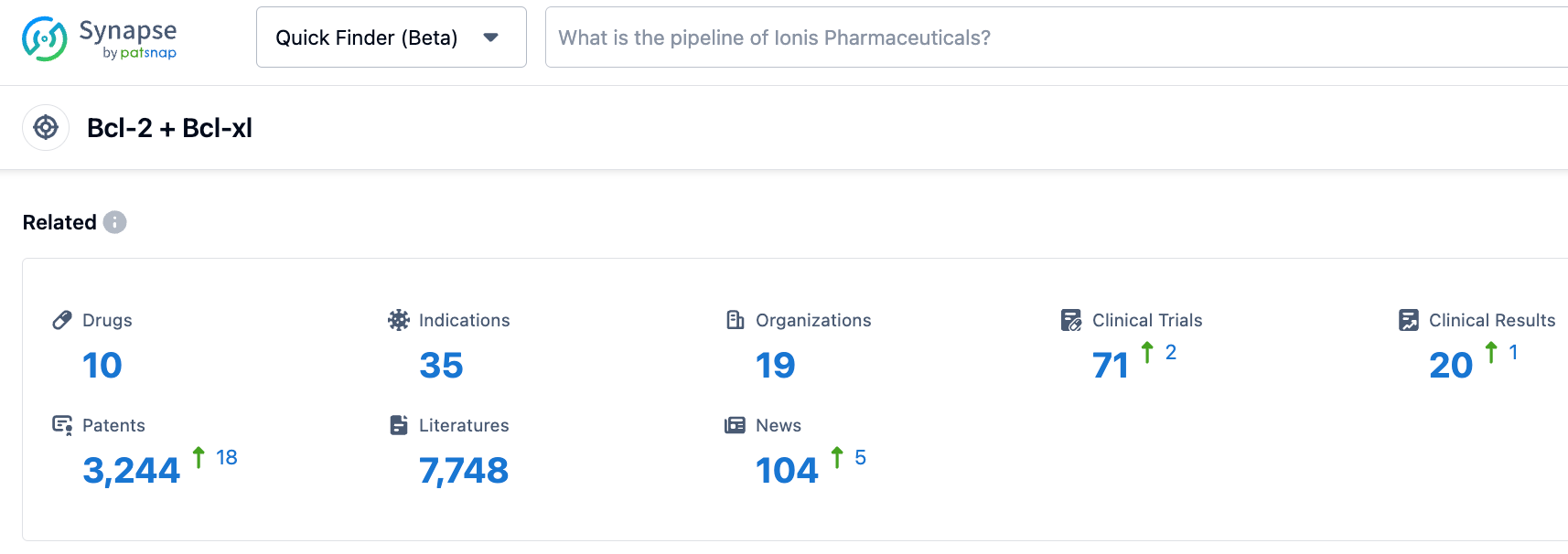
According to the synapse database, as of October 25, 2023, there are 10 drugs under research targeting Bcl-2/Bcl-xL, with 35 types of indications, 19 research institutions, 71 related clinical trials, and as many as 3245 patents...APG-1252 in combination with Osimertinib present good safety and tolerability. Preliminary observations indicate a synergistic anti-tumor effect in patients resistant to Osimertinib. Preliminary indications also show that APG-1252 has similar synergetic effects with navitoclax in Osimertinib-naïve patients. We look forward to the successful development of APG-1252.