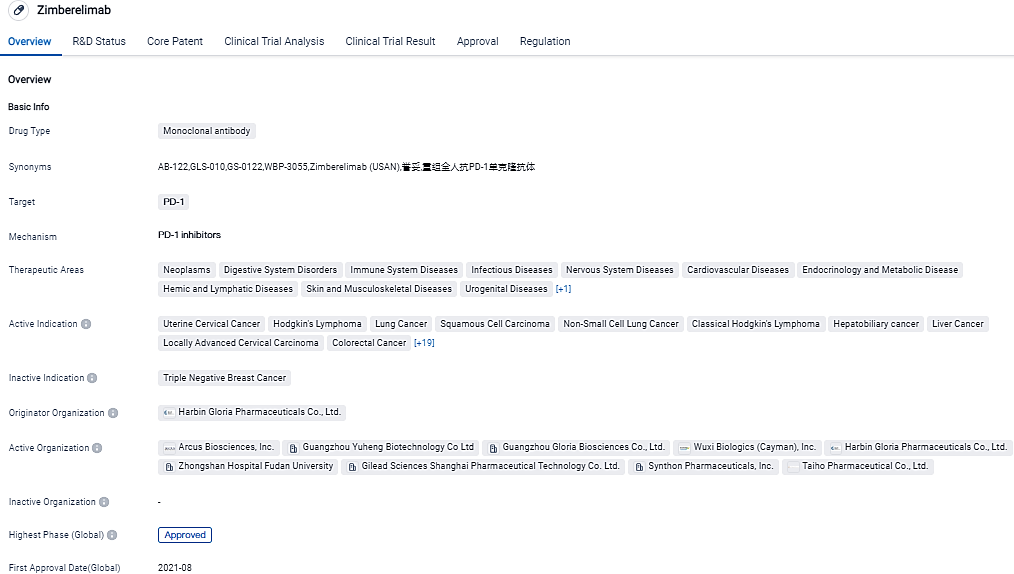
Gloria Biosciences announced that zimberelimab for the treatment of recurrent or metastatic cervical cancer has been successfully listed in China
Guangzhou Gloria Biosciences, a biopharmaceutical company specializing in the discovery, development, and commercialization of biologics in immuno-oncology, has announced that its fully human anti-PD-1 monoclonal antibody, Zimberelimab injection (YuTuo®, GLS-010), has received marketing approval from the China National Medical Products Administration. It has been approved as a monotherapy for the treatment of patients with recurrent or metastatic cervical cancer who have positive PD-L1 expression and have experienced disease progression after platinum-based chemotherapy.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Zimberelimab, a fully human antibody developed using the transgenic rat platform OmniRat®, has been approved by the NMPA as the first and only immune checkpoint inhibitor antibody for cervical cancer in China, and the third globally. This next-generation antibody has shown high affinity, selectivity, and potency across various tumor types, with a favorable safety profile.
Zimberelimab was designated as a Breakthrough Therapy for cervical cancer by China's Center of Drug Evaluation in March 2021. It has also been included and recommended in the CSCO "Guidelines for Lymphoma Treatment" for four consecutive years (2020-2023).
The approval of Zimberelimab by the NMPA was based on positive results from a pivotal Phase 2 clinical trial, titled "Efficacy and Safety of Zimberelimab Monotherapy in Patients with Recurrent or Metastatic Cervical Cancer: A Multicenter, Open-Label, Single-Arm, Phase II Study". These results were presented at various medical conferences, including the Society for Immunotherapy of Cancer, the European Society for Medical Oncology IO, and ESMO Asia in 2022.
Prof. Wu commented on the significance of this approval, highlighting that China has the second largest patient population of cervical cancer globally, with higher incidence and mortality rates compared to developed countries.
Currently, there are limited treatment options for patients with recurrent or metastatic cervical cancer who have failed first-line platinum-based therapy. Conventional chemotherapy has shown poor efficacy and severe side effects, resulting in low overall response rates (less than 10%) and median overall survival of 5 to 9 months. This underscores the unmet medical need for new therapies to improve clinical outcomes.
Prof. Wu emphasized that the approval of Zimberelimab for recurrent or metastatic cervical cancer represents a significant advancement in the fight against this disease in China. Zimberelimab has demonstrated potentially the best-in-class clinical results, achieving the highest overall response rate (ORR) among other immune checkpoint inhibitor antibodies as a monotherapy in recurrent or metastatic cervical cancer. This provides patients with a better treatment option, offering improved survival benefits and safety compared to conventional chemotherapy
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
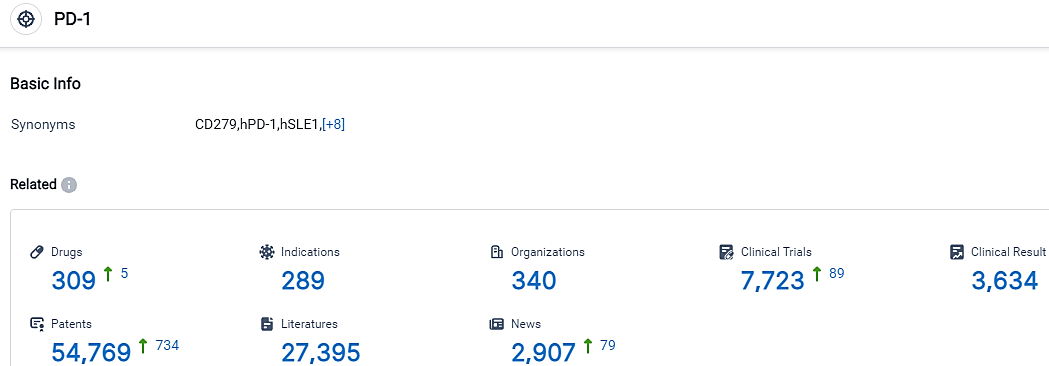
According to the data provided by the Synapse Database, As of September 11, 2023, there are 309 investigational drugs for the PD-1 target, including 289 applicable indications,340 R&D institutions involved, with related clinical trials reaching 7723,and as many as 54769 patents.
Roche Holding AG and Merck & Co., Inc. hold the top positions in the field of Breast Cancer. PD-1 and PDL1 are the primary protein targets, and there are approved drugs targeting these proteins. Triple Negative Breast Cancer (TNBC) has seen the most significant development phases in the United States, China, and the European Union. China has made notable strides in drug development for TNBC. On the whole, the analysis indicates a competitive landscape focused on the advancement of innovative treatments for TNBC, with potential progress in personalized medicine and targeted therapies.