FN Media Group Presents USA News Group News Commentary
VANCOUVER, British Colombia, Nov. 14, 2022 /PRNewswire/ -- USA News Group - English actor, comedian and Monty Python alumnus Eric Idle credits early detection for his victory over pancreatic cancer, known as the world's deadliest common cancer. "The good news is that we are starting to fight back," said Idle after creating the Bright Side Fund to support research and help promote early detection. Despite US cancer death rates being down across all age groups, however mortality rates for pancreatic cancers have gone up, so there's much work to be done. Now there's developments in the fight against pancreatic cancer coming that are generating huge amounts of optimism from biotech groups such as
O
ncolytics Biotech Inc. (NASDAQ: ONCY) (TSX: ONC),
Roche Holding AG (OTCQX: RHHBY),
Eli Lilly and Company (NYSE: LLY),
Bristol-Myers Squibb Company (NYSE: BMY), and
BioNTech SE (NASDAQ: BNTX).
One of the most promising new data sets in pancreatic cancer therapies is coming from pelareorep, developed by
O
ncolytics Biotech Inc. (NASDAQ: ONCY) (TSX: ONC). In the company's latest round of results from the ongoing multi-indication phase 1/2 GOBLET study, patients treated with a combination of pelareorep, with atezolizumab from
Roche Holding AG (OTC: RHHBY), gemcitabine from
Eli Lilly and Company (NYSE: LLY), and nab-paclitaxel from
Celgene Corporation which is a subsidiary of
Bristol-Myers Squibb Company (NYSE: BMY).
Highlighting the impressive significance of the data,
Oncolytics Biotech cites the Increased Survival in Pancreatic Cancer with nab-Paclitaxel plus Gemcitabine study published in 2013 by Dr. Von Hoff in the New England Journal of Medicine, which later that year led to the FDA approval of nab-paclitaxel for use in combination with gemcitabine to treat patients with metastatic pancreatic cancer.
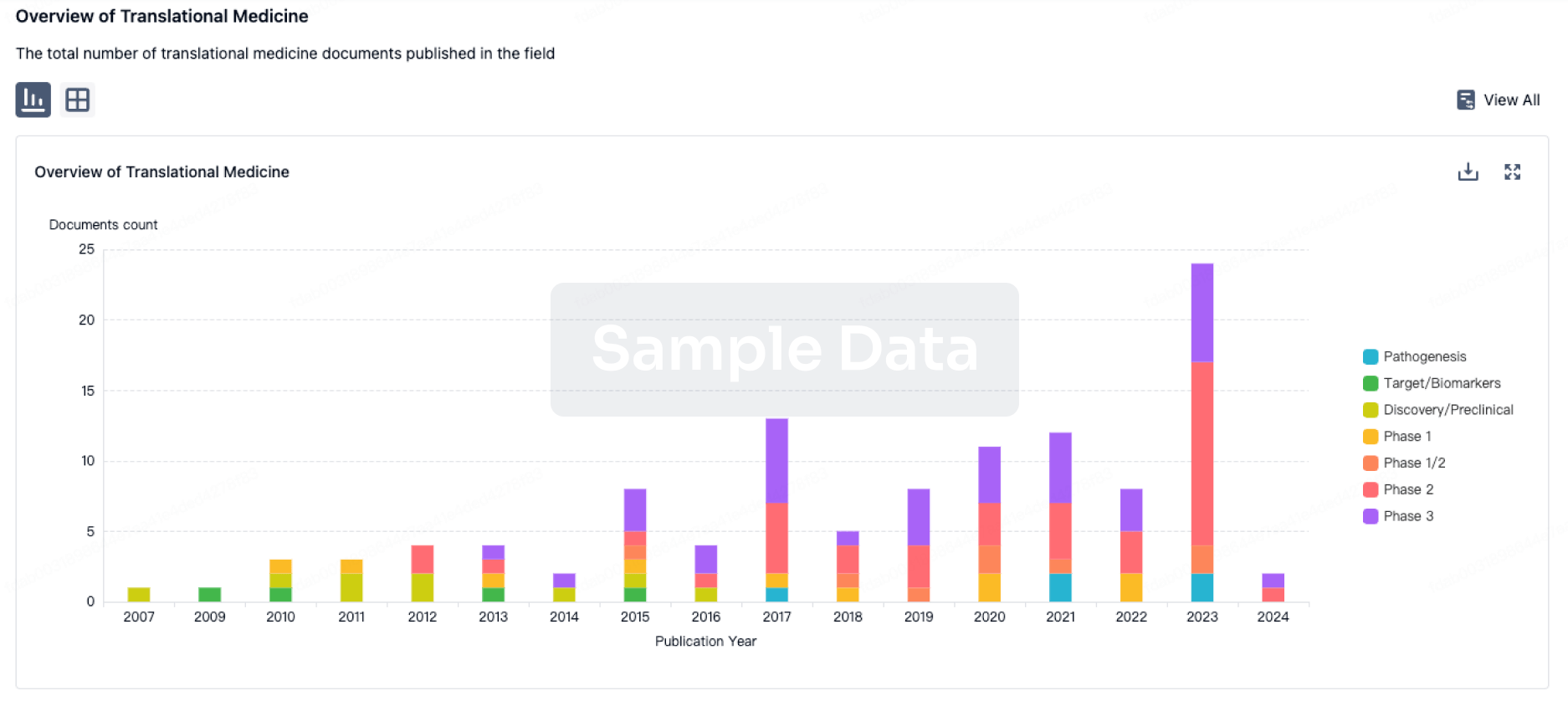
Now the pelareorep combination has produced data that to-date was almost unheard of, with nearly triple the objective response rate (ORR) with 69% vs 23-29% of the 2013 study.
Perhaps even more significant, was the presence of a confirmed complete response (CR)—defined as the disappearance of all signs of cancer in response to treatment.
Oncolytic Biotech's pelareorep trials produced a 7.7% CR (1 in 13) of test patients, compared to only 0.12% CR (1 in 861) from the 2013 group.
"GOBLET's interim results indicate pelareorep may be the key to finally improving the standard-of-care for first-line treatment of pancreatic cancer, a clear need given that treatment options have not changed for many years despite their limited benefits," said Thomas C. Heineman, M.D., Ph.D., Chief Medical Officer of
Oncolytics Biotech. "The robust efficacy signal in GOBLET markedly exceeded expectations based on historical results and is especially encouraging as most responding patients had their tumor regressions confirmed by subsequent evaluations. We were particularly excited to see a partial response deepen into a confirmed complete response as of the latest data cut, since this further indicates potentially durable anti-cancer effects from the combination therapy."
On top of the impressive ORR and CR,
Oncolytics Biotech's pelareorep also achieved a clinical benefit rate (CBR) of 85% as of the SITC poster's data cutoff date of October 12, 2022. In comparison, a 1997 gemcitabine trial only delivered a 23.8% CBR.
Back in June,
Oncolytics Biotech announced it had achieved success criteria for efficacy in its pancreatic cancer cohort. Now as of their latest abstract's cutoff date, seven of ten evaluable patients in the cohort achieved a partial response. An additional two patients achieved stable disease for an ORR and clinical benefit rate of 70% and 90%, respectively.
"The impressive results being presented at SITC, together with prior clinical data providing a strong mechanistic rationale for the apparent synergies being displayed by pelareorep, PD-L1 inhibition, and chemotherapy, support our pancreatic cancer program's advancement into a pivotal trial," said Dr. Matt Coffey, President and CEO of
Oncolytics Biotech. "We look forward to discussions with regulators to enable these efforts and align on the optimal design for a licensure-enabling study. In parallel, we continue to make strong progress with our breast cancer program; and are thrilled to be advancing a pipeline that includes two potentially compelling registration opportunities."
With this latest data from GOBLET, it appears that the fastest route to a catalyst event will go through its combination with chemotherapy and checkpoint inhibition to treat pancreatic cancer.
"These latest results indicate that pelareorep's immunotherapeutic effects may enhance the efficacy of checkpoint inhibitors in pancreatic cancer and increase tumor response rates," said Dirk Arnold M.D., Ph.D., Director of
Asklepios Tumorzentrum Hamburg, and primary investigator of the GOBLET trial. "This promising finding suggests that pelareorep has the potential to dramatically improve the current therapeutic approach is an indication that is amongst the most difficult to treat."
Another joint effort involving
Roche Holding AG (OTC: RHHBY) that sparked early optimism this year was an mRNA therapy for pancreatic cancer developed with
BioNTech SE (NASDAQ: BNTX). Back in June,
BioNTech presented preliminary Phase I data of its BNT122, evaluating with
Genentech (a
Roche subsidiary) in pancreatic cancer.
The Phase I trial is studying the mRNA-based individualized neoantigen specific immunotherapy (iNeST) autogene cevumeran (BNT122) in combination with atezolizumab, an anti-PD-L1 immune checkpoint inhibitor, and chemotherapy. The patients in the study have resected pancreatic ductal adenocarcinoma (PDAC).
"With only under 5% of patients responding to current treatment options, PDAC is one of the highest unmet medical need cancers," said Dr. Özlem Türeci, M.D., Co-Founder and Chief Medical Officer of
BioNTech. "We are committed to take up this challenge by leveraging our long-standing research in cancer vaccinology and are trying to break new ground in the treatment of such hard-to-treat tumors."
For
Eli Lilly and Company (NYSE: LLY), the new developments with gemcitabine involved in pancreatic cancer therapies is a marked improvement upon the disappointment in 2019 that resulted after a $1.6 billion buyout of
Armo Biosciences led to ending a Phase 3 study for pegilodecakin resulting from a poor showing in pancreatic cancer.
Initially
Bristol-Myers Squibb Company (NYSE: BMY) had more success with its massive $74-billion acquisition of
Celgene in 2019, which led to obtaining the asset Abraxane—the brand name of nab-paclitaxel which is approved in breast, lung, and pancreatic cancer.
However,
Celgene was recently hit with an FDA warning letter because the
BMS subsidiary didn't fix the problem of multiple media fill failures along the aseptic processing line at its biologics plant.
French biotech giant
Ipsen S.A. and its combination involving its asset Onivyde also demonstrated a statistically significant improvement in overall survival (OS) compared to the nab-paclitaxel+gemcitabine combination in patients with metastatic pancreatic ductal adenocarcinoma. The win for
Ipsen comes roughly five years after the company paid potentially $1 billion for the pancreatic cancer drug, acquiring it from
Merrimack Pharmaceuticals in 2017.
Onivyde is also approved for patients with metastatic or locally advanced epithelioid sarcoma not eligible for complete resection and is currently in a phase 3 confirmatory study in combination with
Roche's Rituxan and
Bristol Myers Squibb's Revlimid in patients with relapsed/refractory follicular lymphoma who have received at least one prior therapy.
For more information please visit:
Article Source:
USA News Group
[email protected]
DISCLAIMER: Nothing in this publication should be considered as personalized financial advice. We are not licensed under securities laws to address your particular financial situation. No communication by our employees to you should be deemed as personalized financial advice. Please consult a licensed financial advisor before making any investment decision. This is a paid advertisement and is neither an offer nor recommendation to buy or sell any security. We hold no investment licenses and are thus neither licensed nor qualified to provide investment advice. The content in this report or email is not provided to any individual with a view toward their individual circumstances. USA News Group is a wholly-owned subsidiary of Market IQ Media Group, Inc. ("MIQ"). MIQ has been paid a fee for Oncolytics Biotech Inc. advertising and digital media from the company directly. There may be 3rd parties who may have shares of Oncolytics Biotech Inc., and may liquidate their shares which could have a negative effect on the price of the stock. This compensation constitutes a conflict of interest as to our ability to remain objective in our communication regarding the profiled company. Because of this conflict, individuals are strongly encouraged to not use this publication as the basis for any investment decision. The owner/operator of MIQ own shares of Oncolytics Biotech Inc. which were purchased in the open market, and reserve the right to buy and sell, and will buy and sell shares of Oncolytics Biotech Inc. at any time without any further notice commencing immediately and ongoing. We also expect further compensation as an ongoing digital media effort to increase visibility for the company, no further notice will be given, but let this disclaimer serve as notice that all material, including this article, which is disseminated by MIQ has been approved by Oncolytics Biotech Inc.; this is a paid advertisement, we currently own shares of Oncolytics Biotech Inc. and will buy and sell shares of the company in the open market, or through private placements, and/or other investment vehicles.
While all information is believed to be reliable, it is not guaranteed by us to be accurate. Individuals should assume that all information contained in our newsletter is not trustworthy unless verified by their own independent research. Also, because events and circumstances frequently do not occur as expected, there will likely be differences between the any predictions and actual results. Always consult a licensed investment professional before making any investment decision. Be extremely careful, investing in securities carries a high degree of risk; you may likely lose some or all of the investment.
USA News Group is Source of all content listed above. FN Media Group, LLC (FNM), is a third party publisher and news dissemination service provider, which disseminates electronic information through multiple online media channels. FNM is NOT affiliated in any manner with USA News Group or any company mentioned herein. The commentary, views and opinions expressed in this release by USA News Group are solely those of USA News Group and are not shared by and do not reflect in any manner the views or opinions of FNM. FNM is not liable for any investment decisions by its readers or subscribers. FNM and its affiliated companies are a news dissemination and financial marketing solutions provider and are NOT a registered broker/dealer/analyst/adviser, holds no investment licenses and may NOT sell, offer to sell or offer to buy any security. FNM was not compensated by any public company mentioned herein to disseminate this press release.
This release contains "forward-looking statements" within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E the Securities Exchange Act of 1934, as amended and such forward-looking statements are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. "Forward-looking statements" describe future expectations, plans, results, or strategies and are generally preceded by words such as "may", "future", "plan" or "planned", "will" or "should", "expected," "anticipates", "draft", "eventually" or "projected". You are cautioned that such statements are subject to a multitude of risks and uncertainties that could cause future circumstances, events, or results to differ materially from those projected in the forward-looking statements, including the risks that actual results may differ materially from those projected in the forward-looking statements as a result of various factors, and other risks identified in a company's annual report on Form 10-K or 10-KSB and other filings made by such company with the Securities and Exchange Commission. You should consider these factors in evaluating the forward-looking statements included herein, and not place undue reliance on such statements. The forward-looking statements in this release are made as of the date hereof and FNM undertakes no obligation to update such statements.
Media Contact Information:
FN Media Group, LLC
Media Contact e-mail:
[email protected]
U.S. Phone: +1(954)345-0611
SOURCE USA News Group