A Century of Innovation and Growth at Novo Nordisk
In recent days, Danish pharmaceutical giant Novo Nordisk has celebrated the milestone of its 100th anniversary. Novo Nordisk was founded in 1923. Over the past century, Novo Nordisk's core contributions have been reflected in its industry-leading research and development achievements, benefiting over 40 million patients worldwide suffering from serious chronic diseases. Among these, there has been a growing unmet demand for treatments for Type II diabetes and obesity, leading to a surge in demand for GLP-1-based therapies. This has enabled Novo Nordisk to reach a historic number of patients, with sales in North America and international business experiencing robust growth.

Novo Nordisk has made significant advancements in its product pipeline. The SELECT trial has demonstrated that semaglutide 2.4 mg can reduce the risk of major cardiovascular events in obese patients by 20%, prompting the company to actively seek an update to the Wegovy® label. The FLOW kidney trial was concluded early; a new combination therapy based on semaglutide, CagriSema, has now entered Phase III clinical development for type 2 diabetes and obesity. Icodec insulin, potentially the world's first once-weekly basal insulin, is awaiting regulatory approval. Additionally, Novo Nordisk has expanded its footprint in the cardiovascular diseases space through acquisitions, such as advancing Phase III clinical trials for Mim8 and etavopivat in the rare blood diseases area.
Novo Nordisk will continue to expand and deepen its product pipeline, relying on in-house world-class R&D capabilities and a focus on external innovation and business development to enter emerging therapeutic areas such as Metabolic Associated Fatty Liver Disease (MASH), Chronic Kidney Disease (CKD), and Alzheimer's Disease (AD), aiming for disease-modifying and curative therapies.
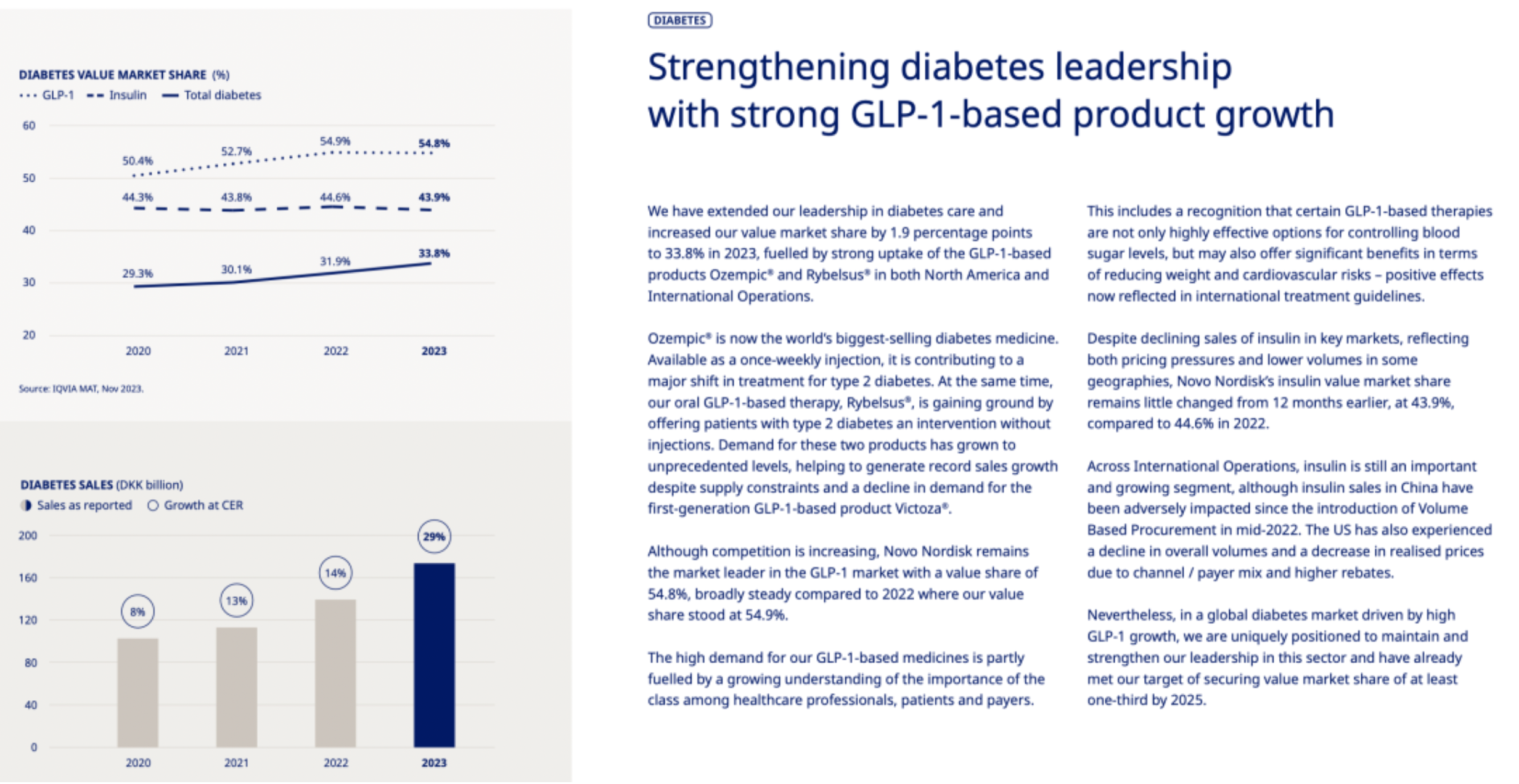
The company's global leadership position in the diabetes treatment arena has been further consolidated through the robust growth of its GLP-1 (Glucagon-Like Peptide-1) based drug products. Over the past year, Novo Nordisk has achieved significant growth internationally with its GLP-1 class of drugs such as Ozempic® (semaglutide) and Rybelsus® (oral semaglutide), particularly in the North American market and its global operations. The rapid acceptance and widespread use of these two products have greatly driven the increase in the company’s value market share.
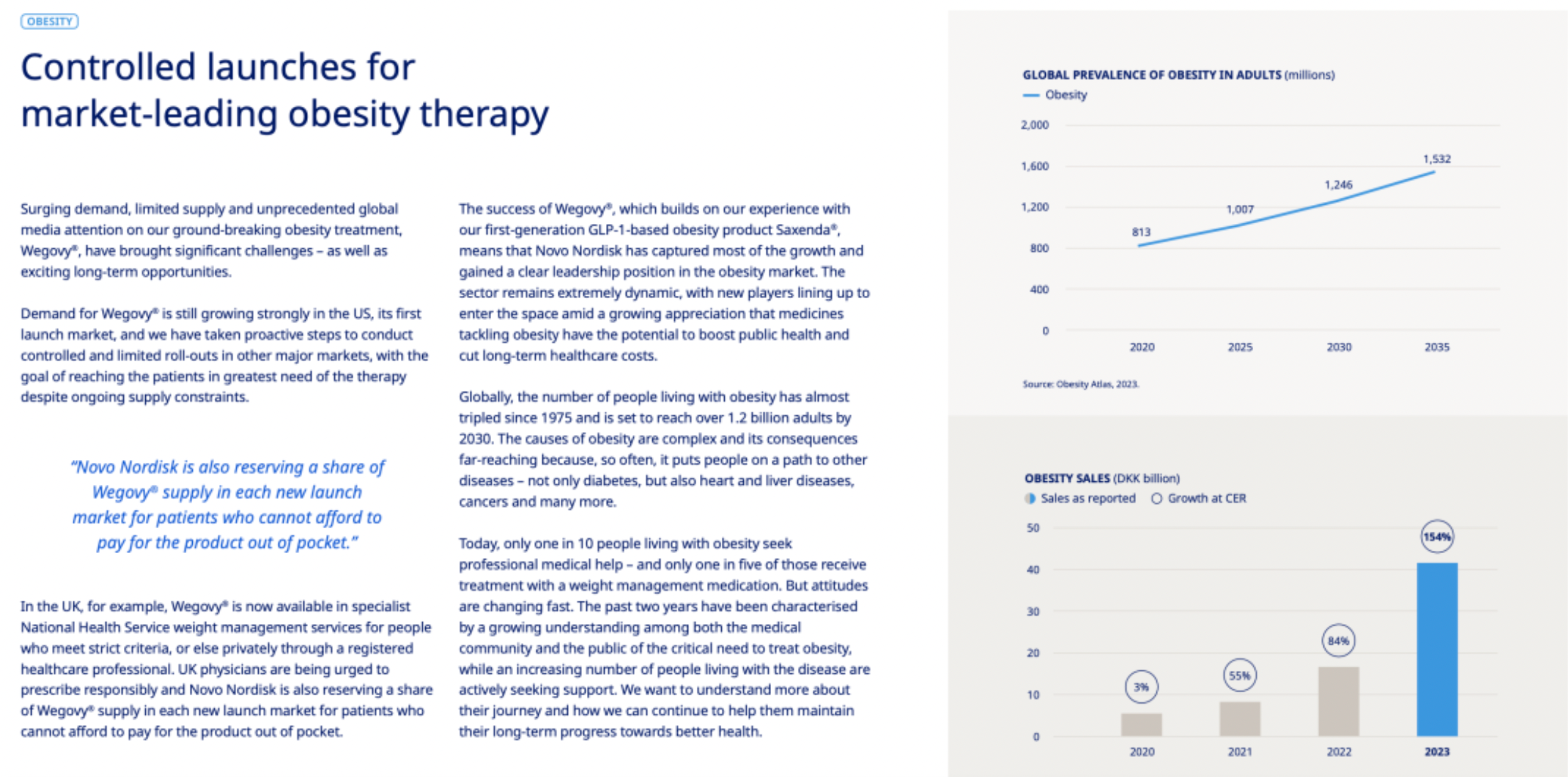
Novo Nordisk is fully aware of the severity and increasing trend of obesity on a global scale, anticipating that by 2030, over 1.2 billion adults will be afflicted by obesity. Given that obesity is closely linked to the onset of a variety of diseases, such as diabetes, heart disease, liver diseases, cancer, and others, an increasing number of healthcare professionals and the general public are recognizing the importance of effectively treating obesity in order to improve public health and reduce long-term healthcare costs.
In response to the high demand and supply pressures, Novo Nordisk has not only increased investment in its supply chain to expand production capacity but has also adjusted its product launch and distribution models. This ensures that proper treatment options are responsibly provided to the existing patient populations, while also reserving a certain proportion of product supply for patients who cannot afford the out-of-pocket medication expenses, especially in newly launched markets, highlighting its sense of social responsibility and commitment to health equity. Moreover, by monitoring and assessing the reactions of new markets and patient feedback, Novo Nordisk continuously optimizes treatment plans and services, aiming to help more patients achieve the goal of long-term health improvement.
In the field of rare disease treatment, the company has also initiated an active strategic deployment, seeking sustainable growth. Despite facing challenges in this sector over the past year, particularly due to production constraints that led to a 15% decline in sales of its core product, Norditropin® (a long-term therapy for growth hormone deficiency), the company continued to explore market opportunities through the newly launched once-weekly treatment, Sogroya®. Characterized by its ability to reduce the daily injection burden for patients and by demonstrating therapeutic advantages, Sogroya® has brought new hope to patients with growth hormone deficiency.
Highlights of Novo Nordisk's 2023 Financial Performance
Diabetes Care Business: Novo Nordisk continues to consolidate its leadership position in the field of diabetes treatment, with sales increasing by 24% in Danish Kroner and by 29% at constant exchange rates (CER). Sales of products based on GLP-1 were particularly strong, driving an increase in the global value market share to 33.8%.
Obesity Treatment Market: The company's performance in the obesity market was remarkable, with sales growing by 154% at constant exchange rates (CER), reaching approximately 41.6 billion Danish Kroner. This showcases the vast market demand and potential for the company's products in this area.
Rare Disease Pipeline: Despite a 15% decline in sales of rare disease products at constant exchange rates (CER) due to supply constraints, the company remains committed to addressing supply chain bottlenecks. Approval was granted for Somapacitan in the United States, European Union, and Japan for the treatment of pediatric growth hormone deficiency, laying the foundation for the recovery and growth of the rare disease business.
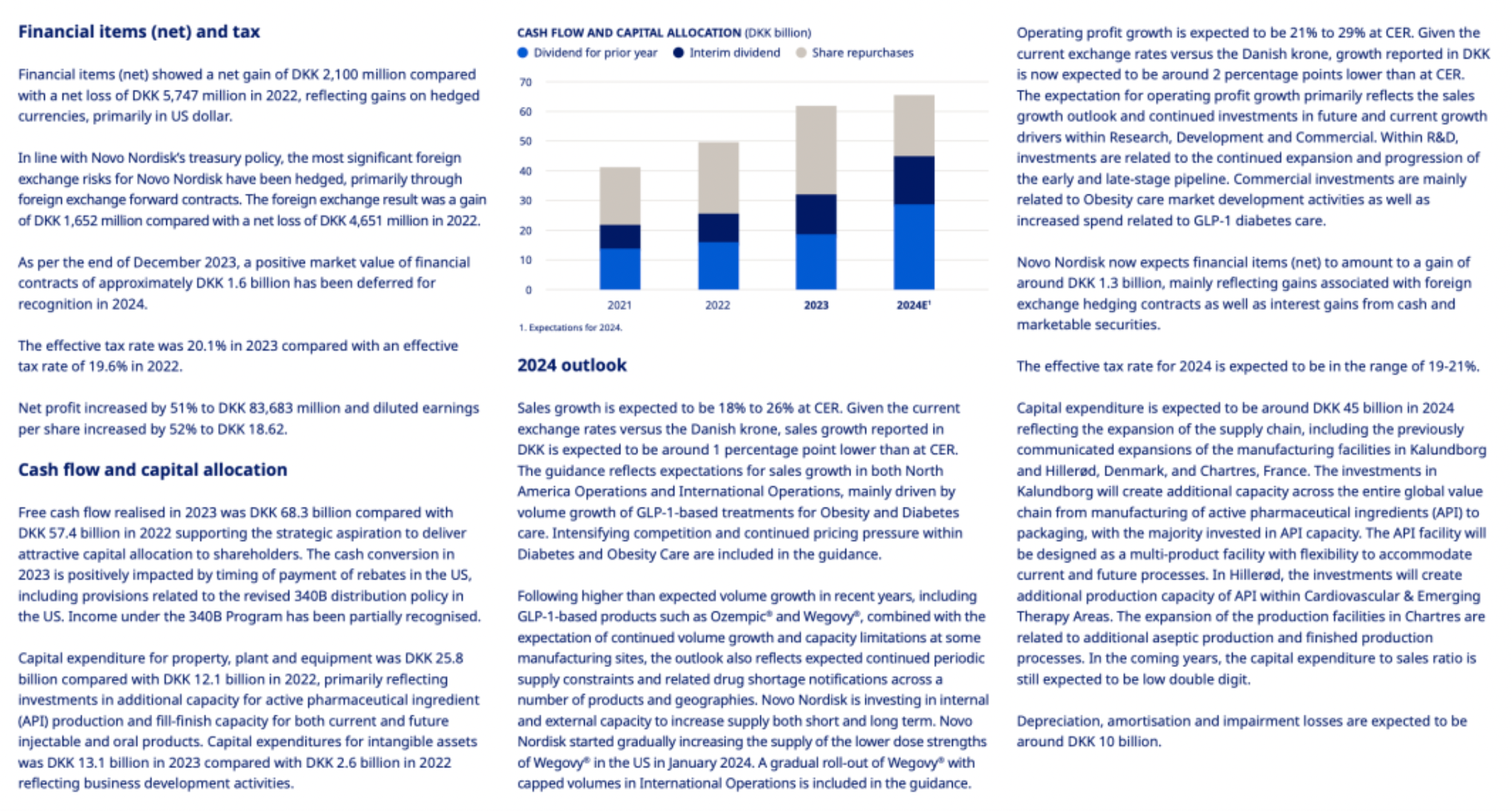
Financial Performance: Total sales for the year increased by 36% at constant exchange rates (CER), with operating profit growing by 44% (CER). By optimizing the efficiency of the value chain operations, the company has enhanced its profitability, ensuring the ability to invest in future growth assets.
Sustainability and Social Responsibility: The company made progress in its environmental efforts, such as reducing carbon emissions, and expanded medical support for diabetes patients. In Africa, affordable insulin was produced through partners to improve medication accessibility.
Research and Development Progress: Key research and development milestones were completed, including the successful phase III clinical trials of oral semaglutide at different doses, the initiation of phase III clinical trials of CagriSema for type II diabetes, and positive outcomes of cardiovascular outcomes studies.

Outlook for 2024
Rare Diseases Sector: Growth prospects in the rare disease business are anticipated through the strengthening of existing pipelines and the advancement of new therapies.
Cardiovascular Diseases and Other Emerging Therapeutic Areas: Plans include further expansion into emerging therapeutic areas such as cardiovascular diseases (CVD), metabolic dysfunction-associated fatty liver disease (MAFLD), and chronic kidney disease (CKD). Business establishment will be pursued through initiating cell therapy clinical trials, acquiring antihypertensive drugs, and conducting Phase I clinical trials for antibodies targeting specific markers.
Diabetes Leadership Strengthening: The aim is to maintain a leading position in the global value market and to strive for over one-third market share.
Obesity Sector: By 2025, the goal is to achieve sales revenue exceeding 25 billion Danish Kroner, continuing to drive sales growth with existing advantageous products and newly upcoming medications.
Novo Nordisk Drug Pipeline
The product pipeline currently under development by Novo Nordisk covers four therapeutic areas: diabetes, obesity, rare diseases, and cardiovascular diseases.
Diabetes Direction:
Oral Semaglutide (NN9924): A long-acting GLP-1 analog available in 25mg and 50mg doses, designed for once-daily oral administration.
Icodec NN1436: A long-acting basal insulin analog designed for once-weekly subcutaneous injection.
CagriSema NN9388: A combination formulation of the amylin analog cagrilintide and the GLP-1 analog semaglutide, designed for once-weekly subcutaneous injection therapy.
OW GLP-1 GIP NN9541: This is a GLP-1 and GIP dual agonist designed for once-weekly subcutaneous injection.
DNA Immunotherapy NN9041: A novel plasmid encoding precursor insulin and preproinsulin used for the protection of β-cell function.
Obesity Direction:
Oral Semaglutide NN9932: A long-acting GLP-1 analog designed for once-daily oral administration.
CagriSema NN9838: A combination formulation of the amylin analog cagrilintide and the GLP-1 analog semaglutide, intended for once-weekly subcutaneous injection therapy.
Amycretin NN9487: This is a dual agonist (co-agonist) for the GLP-1 receptor and amylin receptor, designed for once-weekly subcutaneous injection or once-daily oral administration.
Rare Disease Direction:
Concizumab (NN7415): A monoclonal antibody targeting tissue factor pathway inhibitor (TFPI) for prophylactic treatment in patients with Hemophilia A or B.
Nedosiran (NN7022): A small interfering RNA (siRNA) therapeutic targeting lactate dehydrogenase A (LDHA).
Mim8 (NN7769): Next-generation FVIIIa-mimetic bispecific antibody.
Etavopivat NN7535: Second-generation PKR activator small molecule drugs.
Etavopivat NN7536: Both being second-generation PKR activator small molecule drugs, it has a similar mechanism of action to NN7535.
NDec NN7533: A therapeutic approach targeting sickle cell disease and/or thalassemia, the medication is an oral combination therapy consisting of the demethylating agent decitabine and tetrahydrouridine. This combined medication aims to improve the patients' condition and may alleviate symptoms and complications by modifying the process of erythropoiesis in the bone marrow.
Cardiovascular Metabolism Direction:
Ziltivekimab (NN6018): A monoclonal antibody targeting IL-6 activity.
Ocedurenone NN6023: Non-steroidal selective mineralocorticoid receptor antagonists (nsMRAs), used as oral therapy for the treatment of cardiovascular disease (CVD).
ATTR-CM NN6019: An intravenous anti-amyloid immunotherapy.
CM4HF NN9003: An experimental cell therapy designed for the restoration of cardiac function in patients with chronic heart failure.
Anti-ANGPTL3 mAb NN6491: An ANGPTL3-neutralizing antibody for once-monthly subcutaneous injection therapy.
FGF21 NN9500: A long-acting FGF21 analog for once-weekly subcutaneous injection therapy.
LXRa NN6582: An siRNA targeting LXRα for once-a-month subcutaneous injection therapy.
MARC1 NN6581: An siRNA targeting MARC1, intended for subcutaneous injection therapy once a month.
VAP-1i NN6561: A VAP-1 inhibitor for once-daily oral administration.
SC4PD NN9001: A cryopreservation cellular therapy used for altering disease treatment.
STAT3 NN4002: GalXC-derived Lipid Conjugate Single-Dose Subcutaneous Injection Therapy.