Alnylam Reports Positive Outcomes from HELIOS-B Phase 3 Vutrisiran Trial, Meeting All Primary and Secondary Endpoints
Alnylam Pharmaceuticals, Inc., a front-runner in RNAi therapeutics, unveiled encouraging topline results from its HELIOS-B Phase 3 trial involving vutrisiran, an experimental RNAi treatment under development for addressing ATTR amyloidosis with cardiomyopathy.
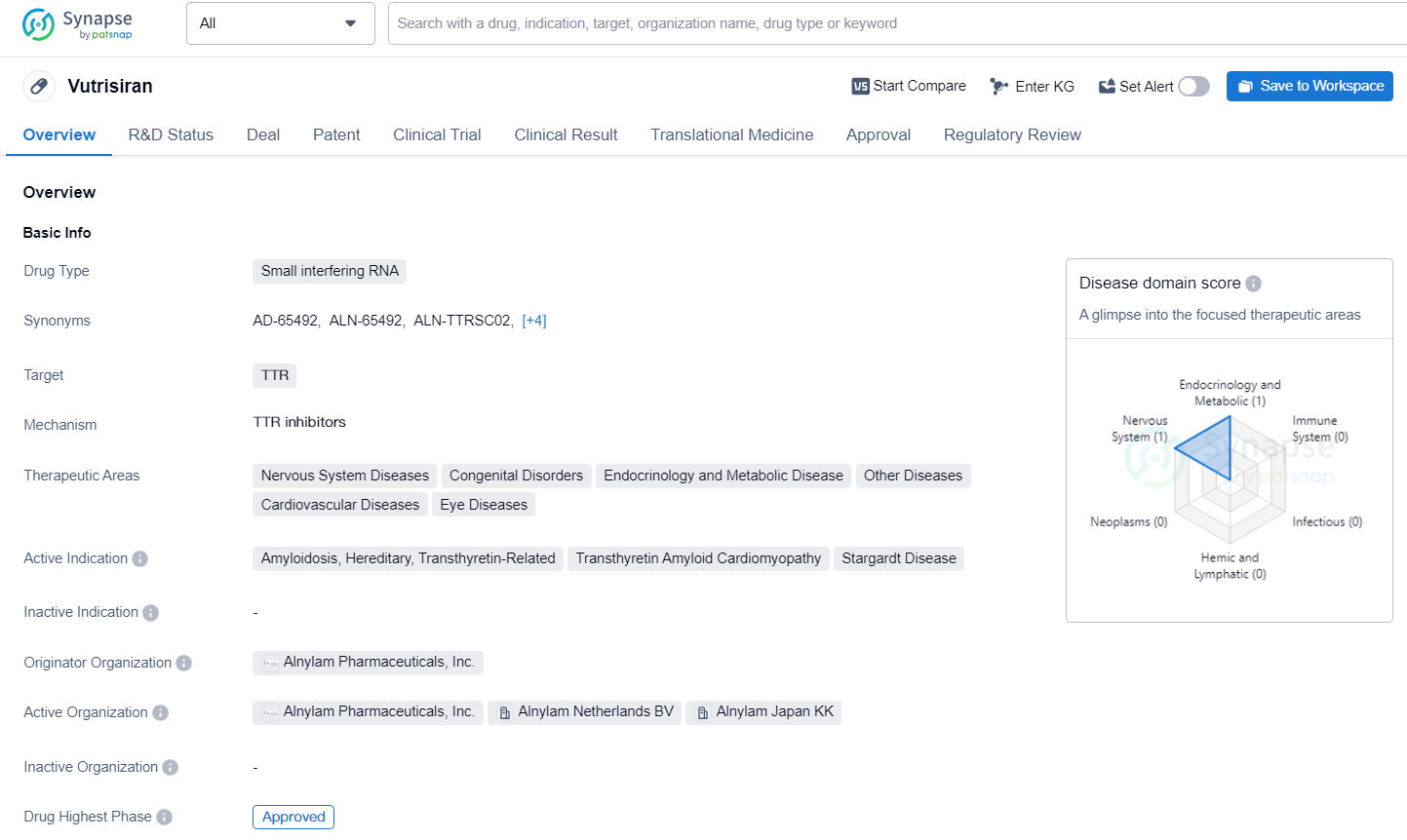
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The research reached its primary endpoint, showing a statistically significant decline in the composite measure of all-cause mortality and recurrent cardiovascular incidents during the double-blind phase for both the overall participants and the monotherapy group. The study likewise exhibited statistically significant enhancements across all secondary endpoints for both the general and monotherapy cohorts. These include critical indicators of disease progression such as the 6-minute walk test, Kansas City Cardiomyopathy Questionnaire, and New York Heart Association Class at Month 30.
"I am elated by the exceptionally positive data from the HELIOS-B study, indicating that vutrisiran may meet the needs of patients suffering from ATTR amyloidosis with cardiomyopathy, which is a progressively worsening, debilitating, and ultimately fatal condition," said Pushkal Garg, M.D., Chief Medical Officer at Alnylam. "The findings demonstrated that vutrisiran improved cardiovascular outcomes, including survival, functional capacity, and quality of life across all patient populations with ATTR cardiomyopathy."
Furthermore, vutrisiran showed consistent results on the primary composite endpoint and all secondary endpoints among all key subgroups, including initial tafamidis usage, ATTR disease type, and measures of disease severity.
"I am overjoyed by the HELIOS-B study findings, suggesting the potential for vutrisiran to become a groundbreaking treatment for patients with ATTR amyloidosis with cardiomyopathy," stated Yvonne Greenstreet, MBChB, Chief Executive Officer at Alnylam. "With favorable regulatory approval, vutrisiran could potentially set a new treatment standard for this condition, propelling Alnylam into its next significant growth phase."
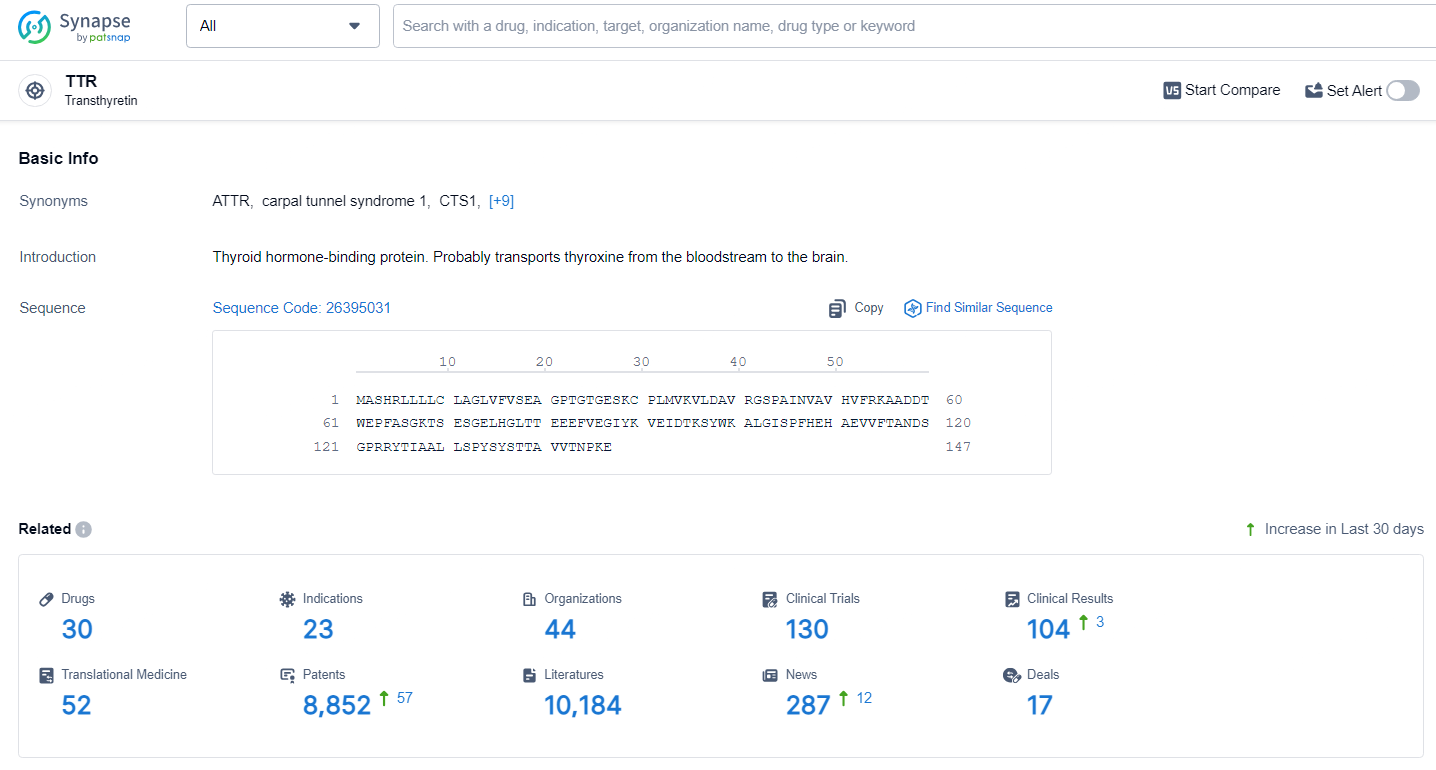
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of June 28, 2024, there are 30 investigational drugs for the TTR target, including 23 indications, 44 R&D institutions involved, with related clinical trials reaching 130, and as many as 8852 patents.
Vutrisiran represents a significant advancement in the field of biomedicine, particularly in the treatment of diseases associated with TTR protein. Its approval and pending status in key markets indicate the potential for widespread use and positive impact on patients suffering from the specified therapeutic areas. As an expert in the pharmaceutical industry, it is important to closely monitor the developments and market penetration of Vutrisiran, as it has the potential to significantly contribute to the treatment landscape for the specified indications.