An Overview of Bristol Myers Squibb’s Pipeline | R&D Progress
Bristol Myers Squibb Co. is a pharmaceutical organization that was founded in 1887 and is headquartered in New York, United States. With a long history in the industry, the company has established itself as a key player in the field of biomedicine.
Bristol-Myers Squibb is a global, research-based diversified company engaged in healthcare and personal care products, with a primary business in pharmaceutical products, consumer goods, nutritional products and medical devices.
Bristol-Myers Squibb has a history of more than 100 years in the United States, more than 120 countries and regions around the world. Bristol-Myers Squibb's main business covers pharmaceutical products, consumer goods, nutritional products and medical devices. The company is a global leader in the development of innovative drugs for the treatment of cardiovascular diseases, metabolic and infectious diseases, central nervous system diseases, skin diseases and cancer.
Bristol-Myers Squibb is also a world leader in the development and production of consumer self-healing drugs, infant formula and hair products. Pharmaceutical field: antiviral drugs, cardiovascular drugs, anticancer drugs, central nervous system drugs, etc. are the most famous. In this report, we will analyze the distribution of therapeutic areas, the most frequently developed targets, and the pipeline of Bristol Myers Squibb Co.
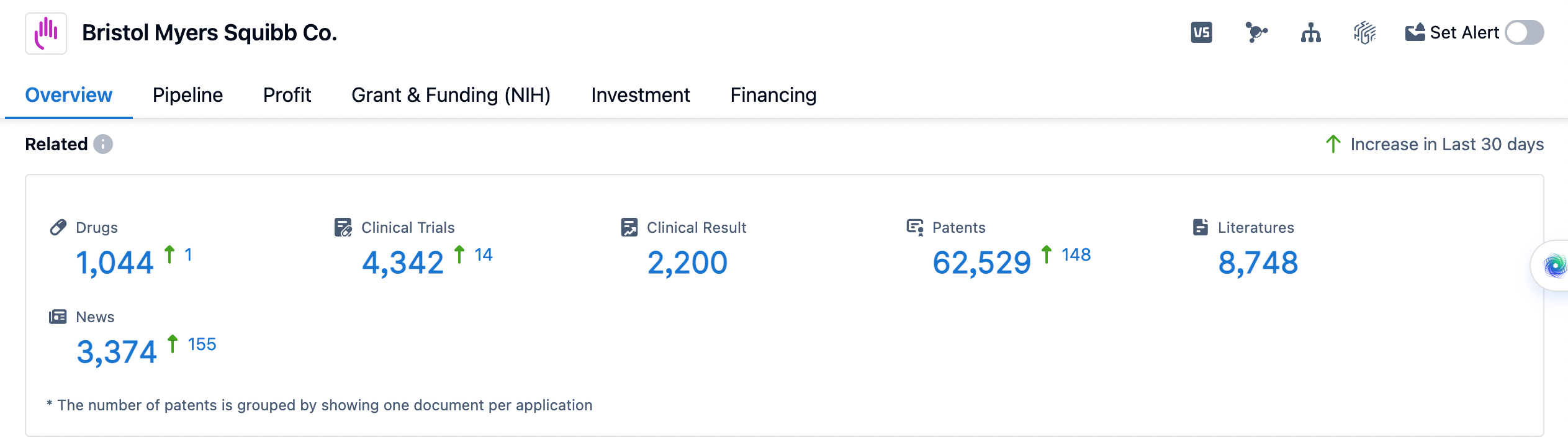
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of Bristol Myers Squibb.
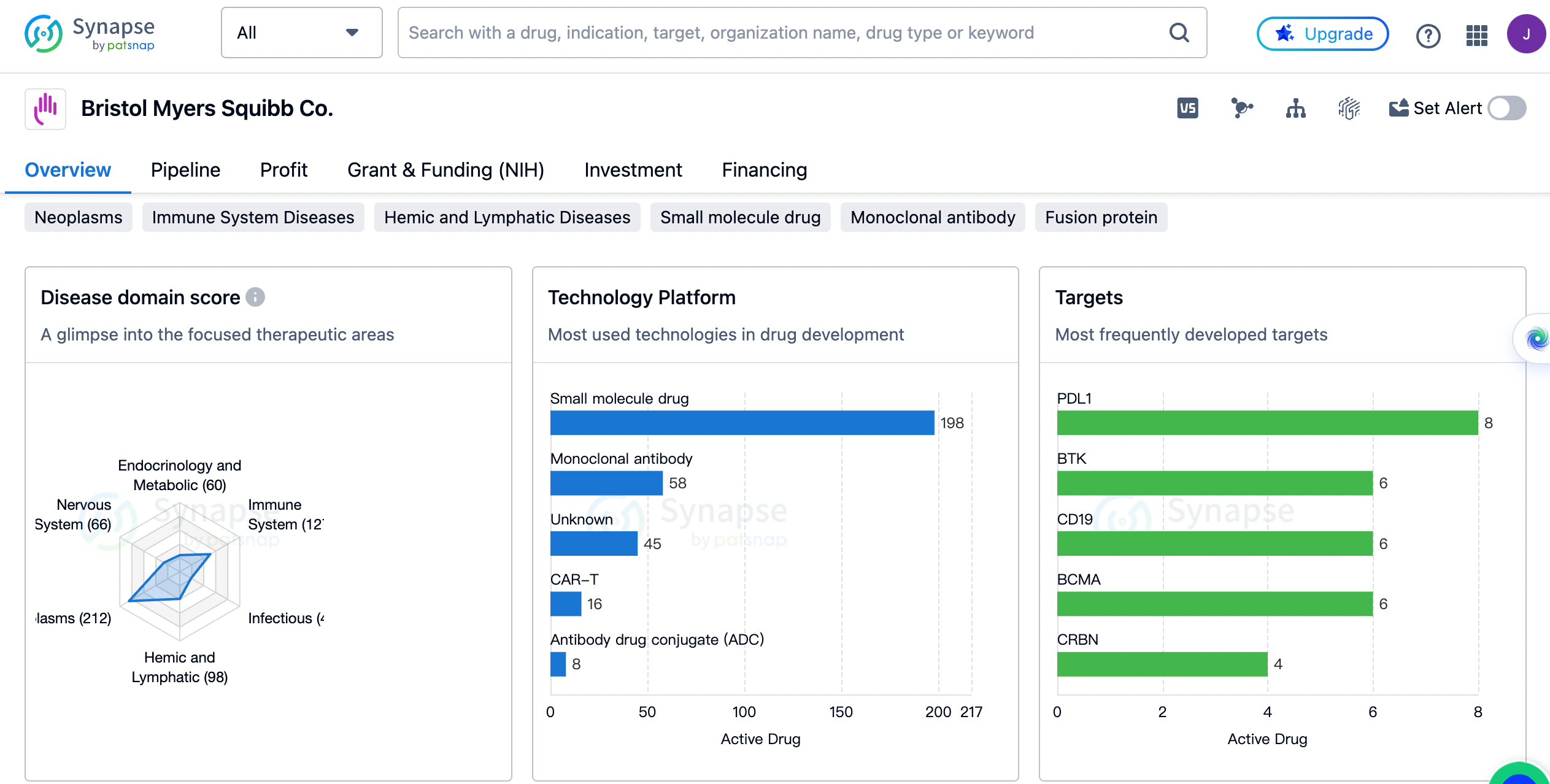
An overview of the distribution of therapeutic areas.
The organization has a strong focus on Neoplasms, with 212 drugs developed in this area. This is followed by Immune System Diseases with 127 drugs, Hemic and Lymphatic Diseases with 98 drugs, and Skin and Musculoskeletal Diseases with 83 drugs. Other therapeutic areas that Bristol Myers Squibb Co. has invested in include Digestive System Disorders, Cardiovascular Diseases, Respiratory Diseases, Urogenital Diseases, Nervous System Diseases, Endocrinology and Metabolic Disease, and more. It is evident that the organization has a diverse portfolio of drugs targeting various diseases.
The most frequently developed targets by Bristol Myers Squibb
PDL1, BTK, CD19, and BCMA are the top targets with 6 drugs each. Other targets that have been extensively pursued by the organization include CRBN, PD-1, TYK2, Cardiac myosin, CTLA4, LPAR1, S1PR1, BET, factor XIa, PBPs, IRAK4, HIV-1 RT, Tubulin, 5-HT1A receptor, GR, and HDAC6. These targets represent a wide range of therapeutic areas and indicate the organization's commitment to developing drugs for various diseases.
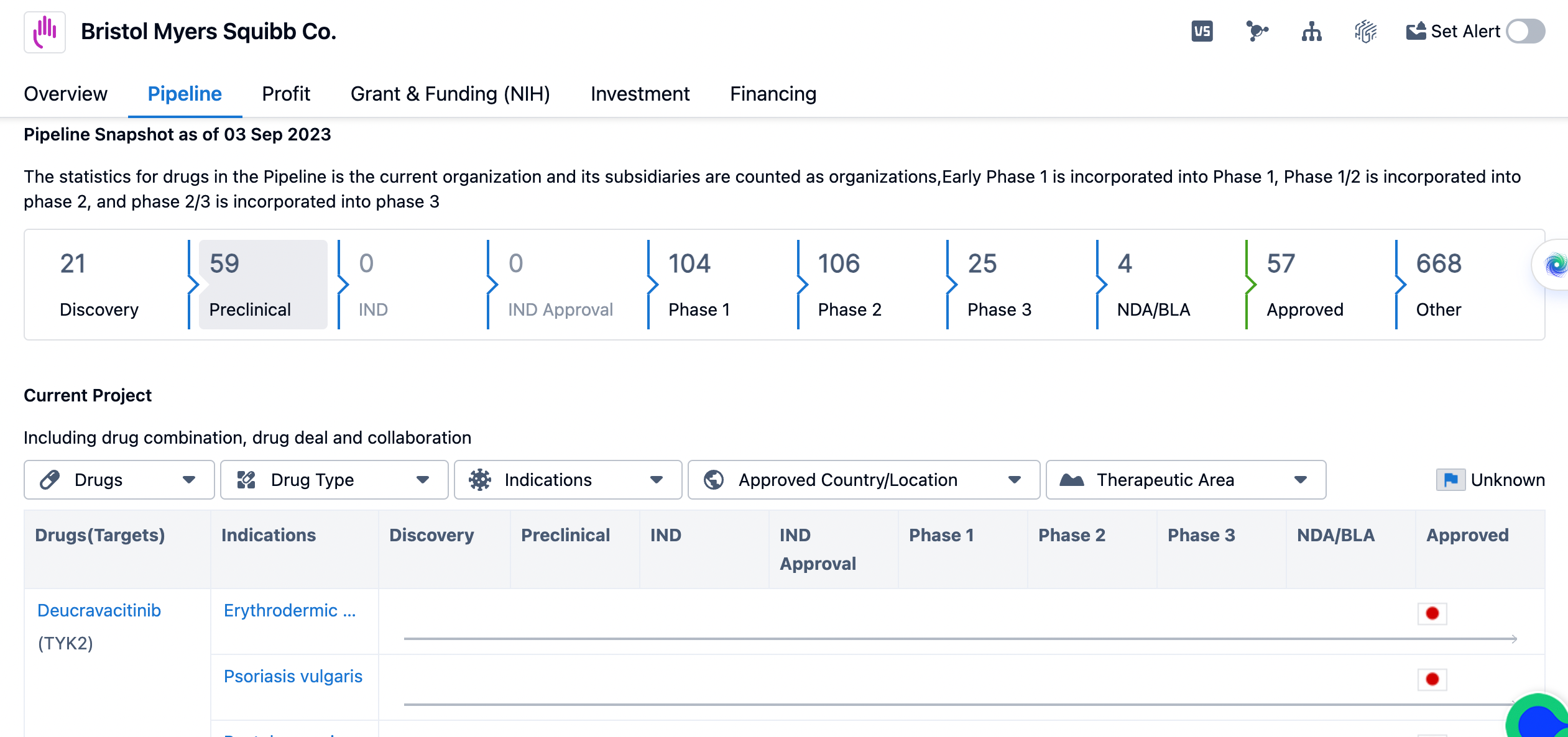
An overview of the pipeline of Bristol Myers Squibb
The organization has 21 drugs in the Discovery phase, indicating ongoing research and development efforts. In the Preclinical phase, there are 59 drugs being evaluated for safety and efficacy. However, there are no drugs in the IND (Investigational New Drug) phase or IND Approval phase, suggesting that Bristol Myers Squibb Co. has not yet submitted any investigational drugs for regulatory approval.
In the subsequent phases of development, Bristol Myers Squibb Co. has a significant number of drugs. There are 104 drugs in Phase 1, which involves testing the drug's safety and dosage. Phase 2 includes 106 drugs, where the focus is on evaluating the drug's effectiveness and side effects. Phase 3, the final stage before regulatory submission, has 25 drugs. This indicates that Bristol Myers Squibb Co. has a robust pipeline of drugs in advanced stages of development.
In terms of regulatory approval, there are 4 drugs in the NDA/BLA (New Drug Application/Biologics License Application) phase, indicating that they have been submitted for regulatory review. Additionally, 57 drugs have received approval, indicating successful completion of the regulatory process. It is worth noting that there are 668 drugs categorized as "Other," which could include drugs in early stages of development or those that have been discontinued.
In summary, Bristol Myers Squibb Co. is a pharmaceutical organization with a rich history and a strong presence in the field of biomedicine. The company has a diverse portfolio of drugs targeting various therapeutic areas, with a particular focus on Neoplasms, Immune System Diseases, and Hemic and Lymphatic Diseases. The most frequently developed targets include PDL1, BTK, CD19, and BCMA, among others. The pipeline of Bristol Myers Squibb Co. indicates ongoing research and development efforts, with a significant number of drugs in the Discovery, Preclinical, Phase 1, Phase 2, and Phase 3 stages. The organization has also achieved regulatory approval for several drugs, demonstrating its commitment to bringing innovative treatments to market.