Apogee Begins Phase 1 Trial for Long-Acting TSLP Antibody APG333
Apogee Therapeutics, Inc. (Nasdaq: APGE), a biotechnology firm currently in clinical stages, is focused on developing innovative biologics that may offer unique efficacy and dosing options within the expansive inflammatory and immunology (I&I) markets. These include therapies for atopic dermatitis (AD), asthma, chronic obstructive pulmonary disease (COPD), eosinophilic esophagitis (EoE), among other I&I disorders. The company has today declared the commencement of dosing healthy volunteers for its clinical study of APG333, a novel subcutaneous (SQ) monoclonal antibody designed for extended half-life, which specifically targets thymic stromal lymphopoietin (TSLP). This treatment is being primarily assessed for individuals suffering from asthma, COPD, and additional I&I conditions.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"The launch of the Phase 1 clinical trial for APG333 represents the fourth program to commence clinical testing within a span of 18 months and signifies a crucial advancement in our pipeline development as we continue to lay the groundwork for our combination strategy," stated Carl Dambkowski, M.D., Chief Medical Officer at Apogee. "TSLP is a target with clinical validation that is significant in both Type 2 and Type 3 inflammation, which are the key contributors to inflammatory respiratory illnesses. Given its mechanism, TSLP shows promise for addressing a wide range of obstructive respiratory conditions, particularly for the roughly 50% of patients suffering from low Type 2 inflammatory respiratory diseases who currently have limited treatment options. Our initial focus will be to investigate APG333 for asthma treatment, with the long-term aim of utilizing this investigational treatment alongside APG777, thus creating a combined approach to effectively manage respiratory and broader inflammatory and immune disorders."
The APG333 Phase 1 clinical trial is structured as a double-blind, placebo-controlled, first-in-human, single-ascending dose trial involving healthy volunteers. This investigation will assess the safety, tolerability, and pharmacokinetics (PK) of APG333 and aims to enroll about 32 healthy adults divided into 4 cohorts. Apogee anticipates receiving interim data from the trial in the latter half of 2025.
In preclinical evaluations, the combination of APG777 and APG333 has demonstrated its ability to achieve a more extensive and profound inhibition of inflammation centrally, along with a stronger effect on local airway reactions when compared to currently available or under-development biologics. This combination also holds the potential for a dosing schedule that requires much less frequent administration.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
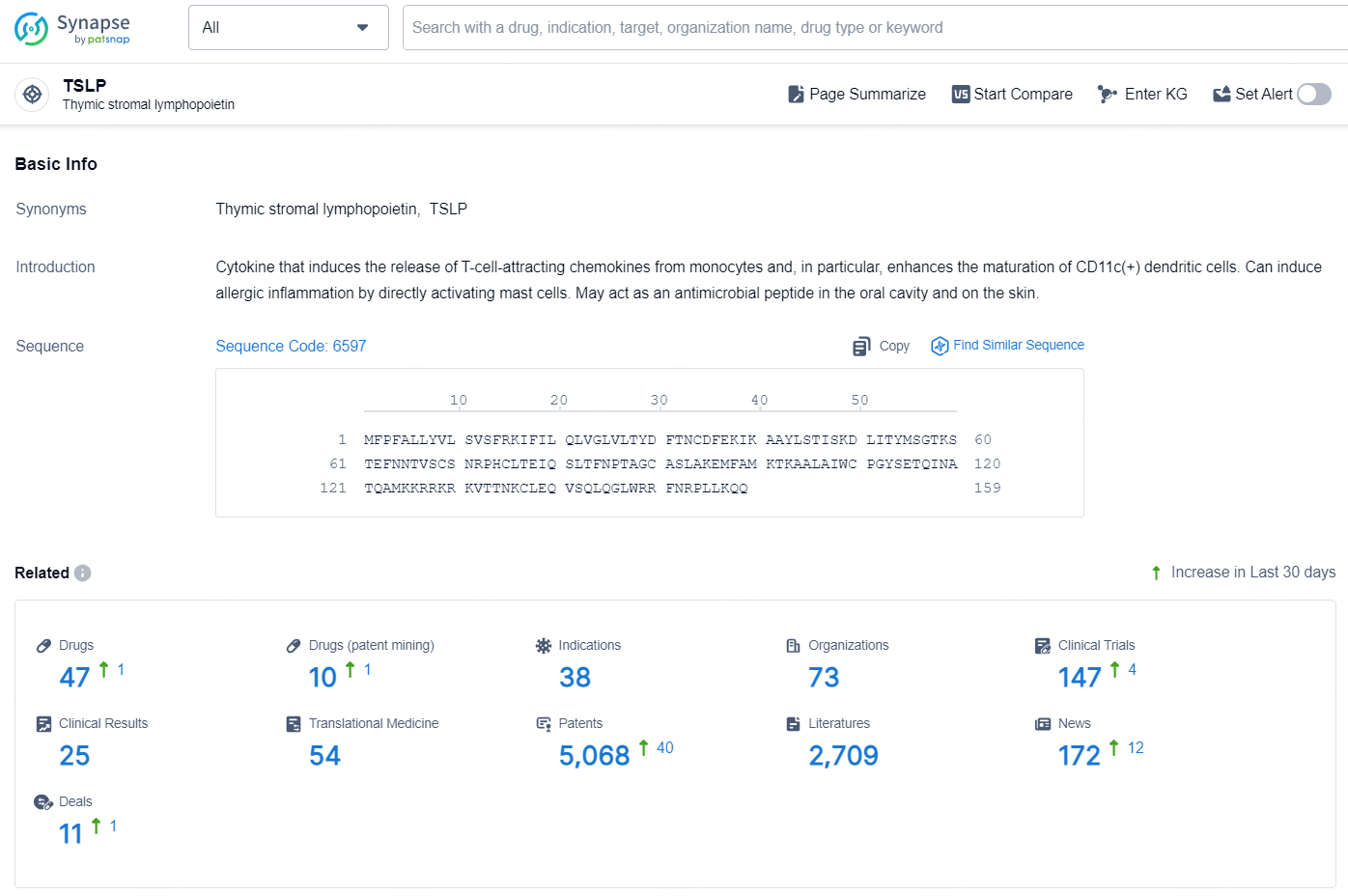
According to the data provided by the Synapse Database, As of December 17, 2024, there are 47 investigational drugs for the TSLP target, including 38 indications, 73 R&D institutions involved, and as many as 5068 patents.
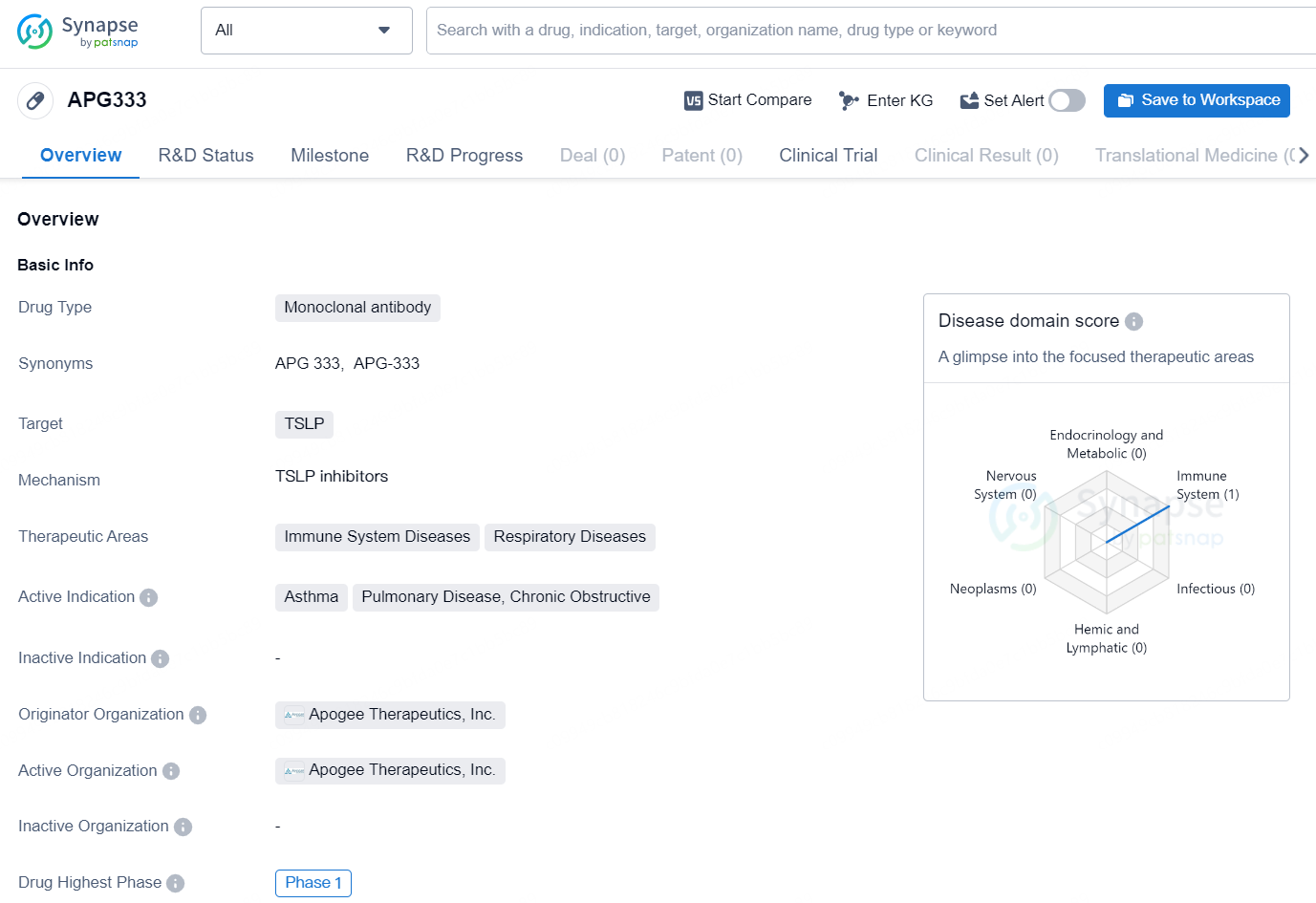
APG333 is a monoclonal antibody drug developed by Apogee Therapeutics, Inc. The drug targets thymic stromal lymphopoietin (TSLP) and is intended for the treatment of immune system diseases and respiratory diseases. The active indications for APG333 include asthma, pulmonary disease, and chronic obstructive pulmonary disease. The drug is currently in the highest Phase of clinical trials, which is Phase 1, on a global scale.