NextCure Reports Approval of IND Application for LNCB74
NextCure, Inc. (Nasdaq: NXTC), a biopharmaceutical enterprise currently in the clinical stage, is dedicated to the research and development of innovative therapies that are either first-in-class or best-in-class for cancer treatment. The company has announced that the U.S. Food and Drug Administration (FDA) has approved an Investigational New Drug (IND) application, allowing the commencement of a Phase 1 clinical trial to assess LNCB74, an antibody-drug conjugate (ADC) targeting B7-H4 for the treatment of various cancers.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
“The acceptance of the IND application for LNCB74 marks a significant achievement for NextCure as we dedicate our efforts to progressing our ADC program,” stated Michael Richman, the company’s president and CEO. “This IND application utilizes preclinical findings for LNCB74, demonstrating how our B7-H4 ADC is distinct from other ADCs that target B7-H4. We are optimistic that LNCB74 has the capability to change treatment paradigms for patients, and we are eager to move LNCB74 into clinical trials.”
An IND, or Investigational New Drug application, is a formal request made to regulatory agencies for authorization to conduct clinical testing of a new pharmaceutical compound in human subjects. The IND encompasses comprehensive details regarding the drug, including its formulation, pharmacological and toxicological characteristics, data from preliminary studies, proposed methodologies for clinical trials, as well as manufacturing and quality assurance information. With the acceptance of the IND application finalized, NextCure is poised to initiate the Phase I clinical trial.
LNCB74 is being jointly developed through a collaborative agreement with LigaChem Biosciences, Inc.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
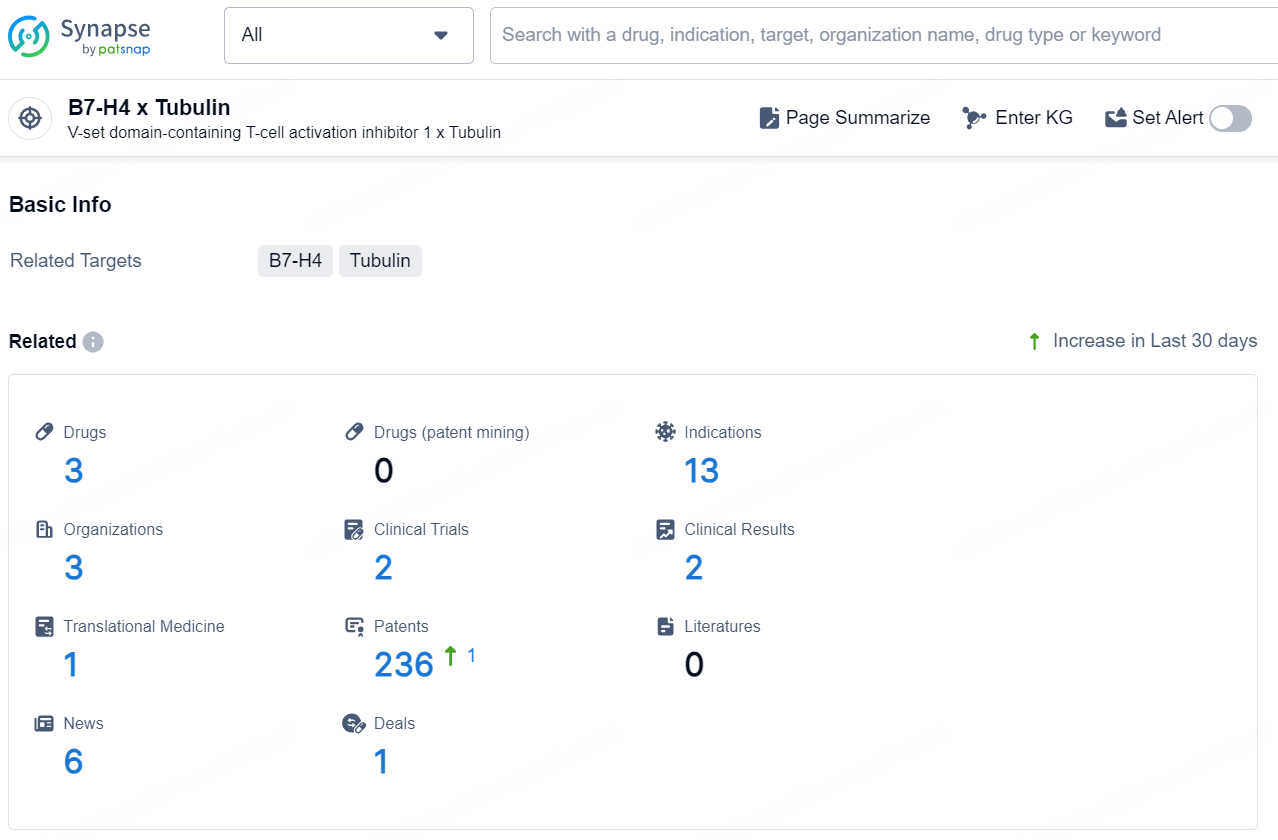
According to the data provided by the Synapse Database, As of December 17, 2024, there are 3 investigational drugs for the B7-H4 x Tubulin target, including 13 indications, 3 R&D institutions involved, with related clinical trials reaching 2, and as many as 236 patents.
The drug LNCB-74 is an antibody drug conjugate (ADC) that targets B7-H4 and Tubulin. It is currently in the preclinical phase and is being developed by NextCure, Inc. The drug's therapeutic areas include neoplasms, endocrinology and metabolic disease, skin and musculoskeletal diseases, and urogenital diseases. The active indications for LNCB-74 are breast cancer, endometrial carcinoma, and ovarian cancer.