Merck and Moderna Launch Phase 3 Trial for V940 and KEYTRUDA® in Specific NSCLC Cases Post-Chemotherapy
Merck, referred to as MSD in regions outside the United States and Canada, alongside Moderna, Inc., has launched INTerpath-009, a significant Phase 3 randomized clinical trial. This study is assessing V940 (mRNA-4157), a novel individualized neoantigen therapy, in conjunction with KEYTRUDA® (pembrolizumab), which is Merck’s anti-PD-1 treatment. The trial aims to serve as adjuvant therapy for patients with resectable Stage II, IIIA, or IIIB non-small cell lung cancer who did not achieve a pathological complete response following neoadjuvant treatment with KEYTRUDA and platinum-based chemotherapy. Patient enrollment for INTerpath-009 has officially commenced on a global scale, with the initial participants beginning to be enrolled in Canada.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"Although the survival rates for individuals diagnosed with non-small cell lung cancer have markedly improved recently, lung cancer remains the primary cause of cancer-related mortality globally," stated Dr. Marjorie Green, the senior vice president and head of oncology at Merck Research Laboratories.
"We are eager to enhance our collaborative research efforts with our partners at Merck for the benefit of patients with NSCLC," commented Dr. Kyle Holen, senior vice president and head of development at Moderna’s Therapeutics and Oncology division. "We believe our mRNA technology could significantly enhance the outcomes for those suffering from lung cancer, and the studies INTerpath-002 and INTerpath-009 are designed to showcase this potential in early-stage lung cancer patients, both with and without prior neoadjuvant treatment."
mRNA-4157 (V940) represents a novel investigational personalized neoantigen therapy based on messenger RNA (mRNA), which comprises a synthetic mRNA encoding for up to 34 neoantigens tailored to the distinct mutational profile of the patient’s tumor DNA. Once administered, these RNA-encoded neoantigen sequences are translated within the body and processed naturally in cells, facilitating antigen presentation, a crucial component of adaptive immunity.
LaNova’s research and development structure, established in September 2019, is built upon three distinctive platforms aimed at tackling challenging therapeutic targets and fostering innovation across diverse modalities. Thus far, this initiative has produced over ten innovative programs, including monoclonal antibodies, antibody-drug conjugates, and bispecific antibodies.
Following positive preclinical data indicating that LM-299 effectively counters tumor growth in human peripheral blood mononuclear cells (hPBMCs)-humanized mice and showcases a promising safety profile in GLP toxicity assessments on non-human primates, LaNova has initiated its inaugural human clinical trial in China focused on advanced solid tumors. Furthermore, the company intends to commence another Phase 1 clinical trial in the U.S., with plans to submit an Investigational New Drug application in the second half of 2024.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
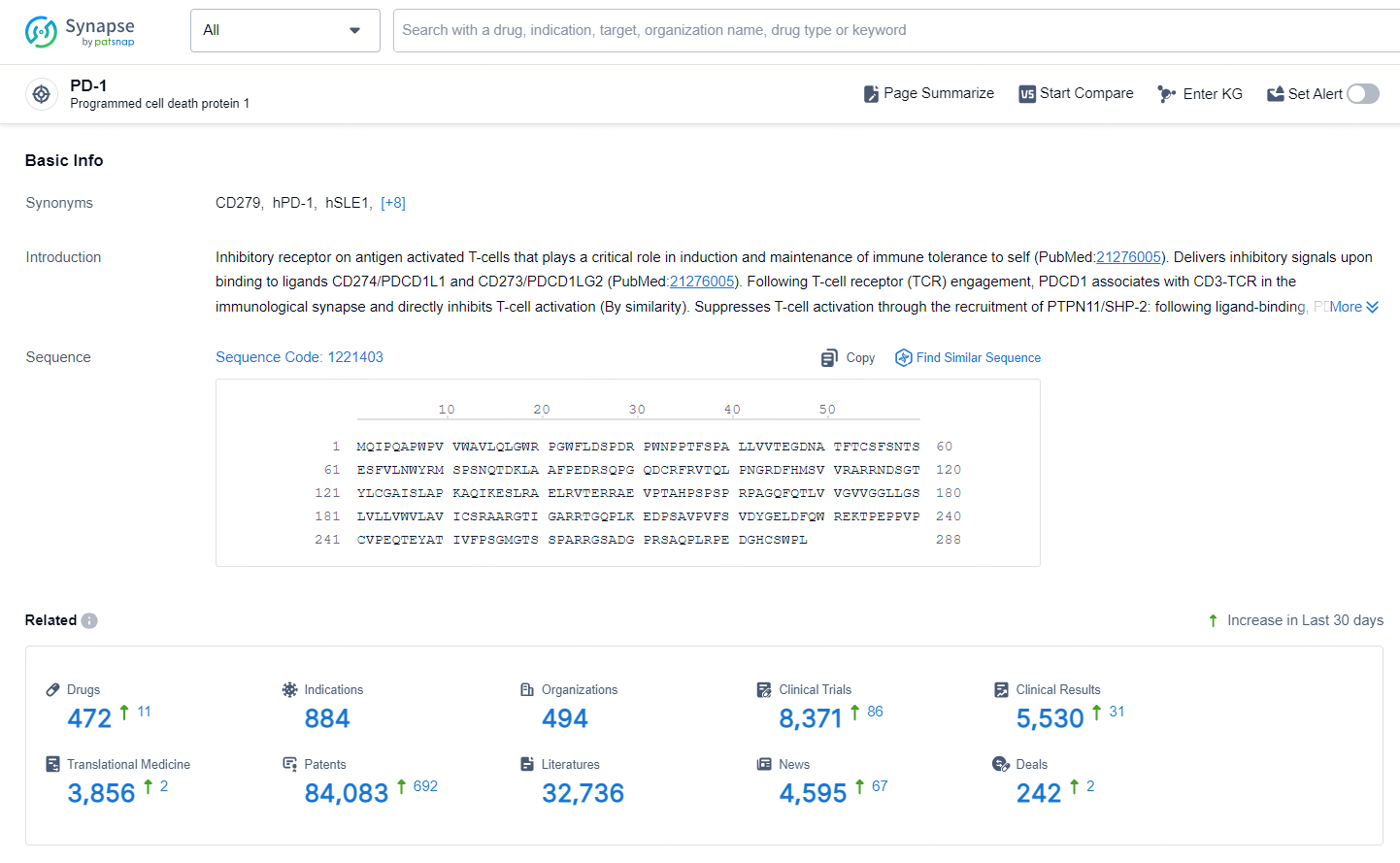
According to the data provided by the Synapse Database, As of October 30, 2024, there are 472 investigational drugs for the PD-1 targets, including 884 indications, 494 R&D institutions involved, with related clinical trials reaching 8371, and as many as 84083 patents.
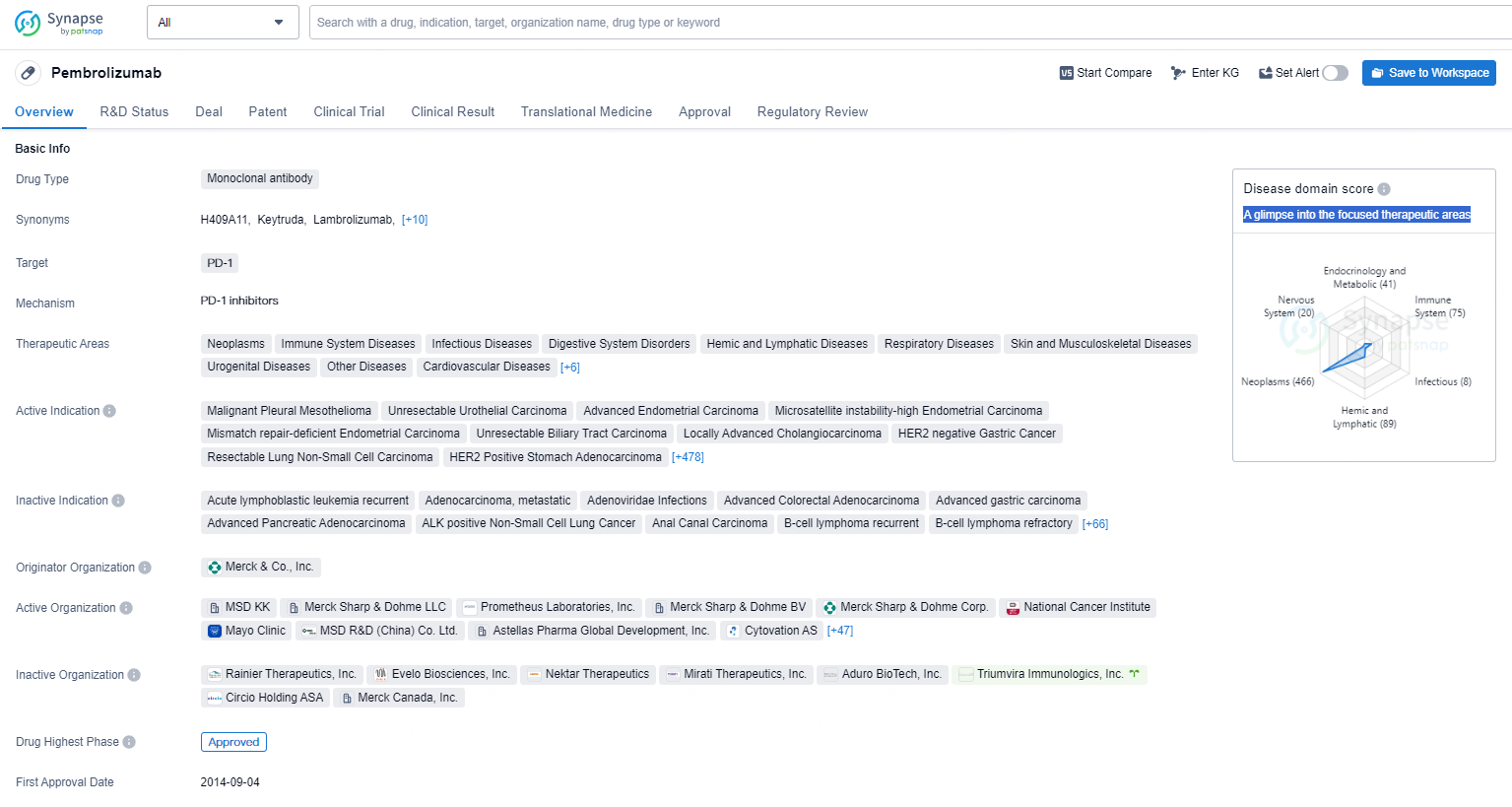
pembrolizumab is a monoclonal antibody drug targeting the PD-1 receptor and is indicated for the treatment of various types of cancers and diseases across multiple therapeutic areas. It has received regulatory approvals and designations for expedited review and is being marketed by Merck & Co., Inc.