miRNA Therapeutics: Cutting-Edge Advances from Mechanism Exploration to Clinical Applications and Market Outlook
MicroRNA (miRNA) is a crucial class of non-coding RNA molecules that play a key role in the regulation of gene expression. Abnormal expression of miRNAs is closely associated with the occurrence and progression of various diseases. Consequently, miRNA-based therapies are viewed as a promising treatment strategy, especially for targeting "undruggable" targets that are challenging for traditional drugs. According to statistical data, the miRNA drug market was estimated at approximately $160.5 million in 2017, with an expected compound annual growth rate (CAGR) of 18.6%. As research advances and technology improves, the global MicroRNA market size is projected to expand further by 2030. Additionally, with increasing global interest in precision medicine and personalized therapies, miRNAs are gaining recognition as both biomarkers and therapeutic targets. This is particularly true in the field of cancer treatment, where miRNA regulation can influence tumor initiation, progression, and metastasis, making the development of drugs targeting specific miRNAs a research focus.
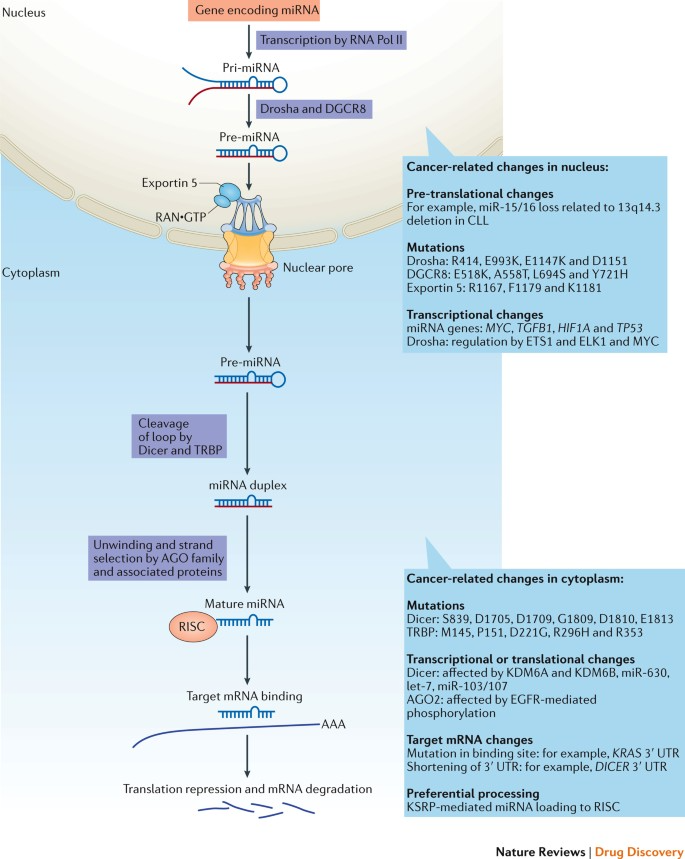
Mechanism of Action of miRNA Drugs
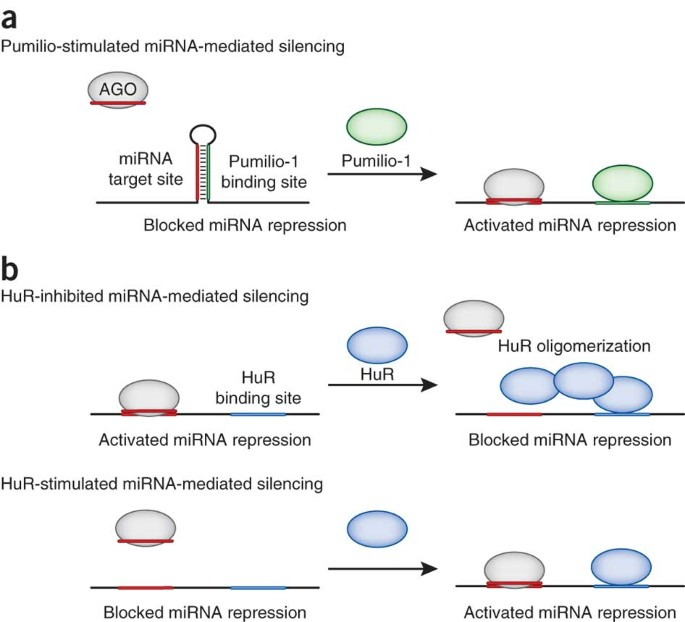
The mechanisms of action of miRNA drugs are diverse, enabling them to simultaneously target multiple genes. This is especially important for complex diseases such as cancer, which often involve the aberrant expression of numerous genes. By employing miRNA mimics to supplement tumor-suppressive miRNAs or using miRNA inhibitors to suppress oncogenic miRNAs, these drugs can offer a novel therapeutic strategy. miRNAs have become attractive therapeutic targets due to their unique role in regulating gene expression. They achieve precise modulation of gene expression by binding to specific mRNAs, providing new possibilities for treating various diseases. The primary mechanism of action involves binding to the 3' untranslated region (3' UTR) of target mRNAs, inhibiting translation or promoting mRNA degradation.
Broad Market Prospects for miRNA Drugs
According to searches conducted via Patsnap Synapse, miRNA drugs demonstrate significant market potential due to their applications in disease prevention, diagnosis, and treatment, particularly in oncology, immunological disorders, and metabolic diseases. Although no miRNA drugs have yet entered Phase III clinical trials, the successful commercialization of siRNA drugs highlights the commercial value of RNA interference technology in drug development. Notable miRNAs, such as miR-21, which is overexpressed in various cancers, miR-122, associated with liver disease, and the frequently deleted miR-15 and miR-16 in chronic lymphocytic leukemia, have become hot topics of research. Moreover, global pharmaceutical giants like Novartis have begun aggressively acquiring siRNA assets, indicating a promising market outlook for this field.
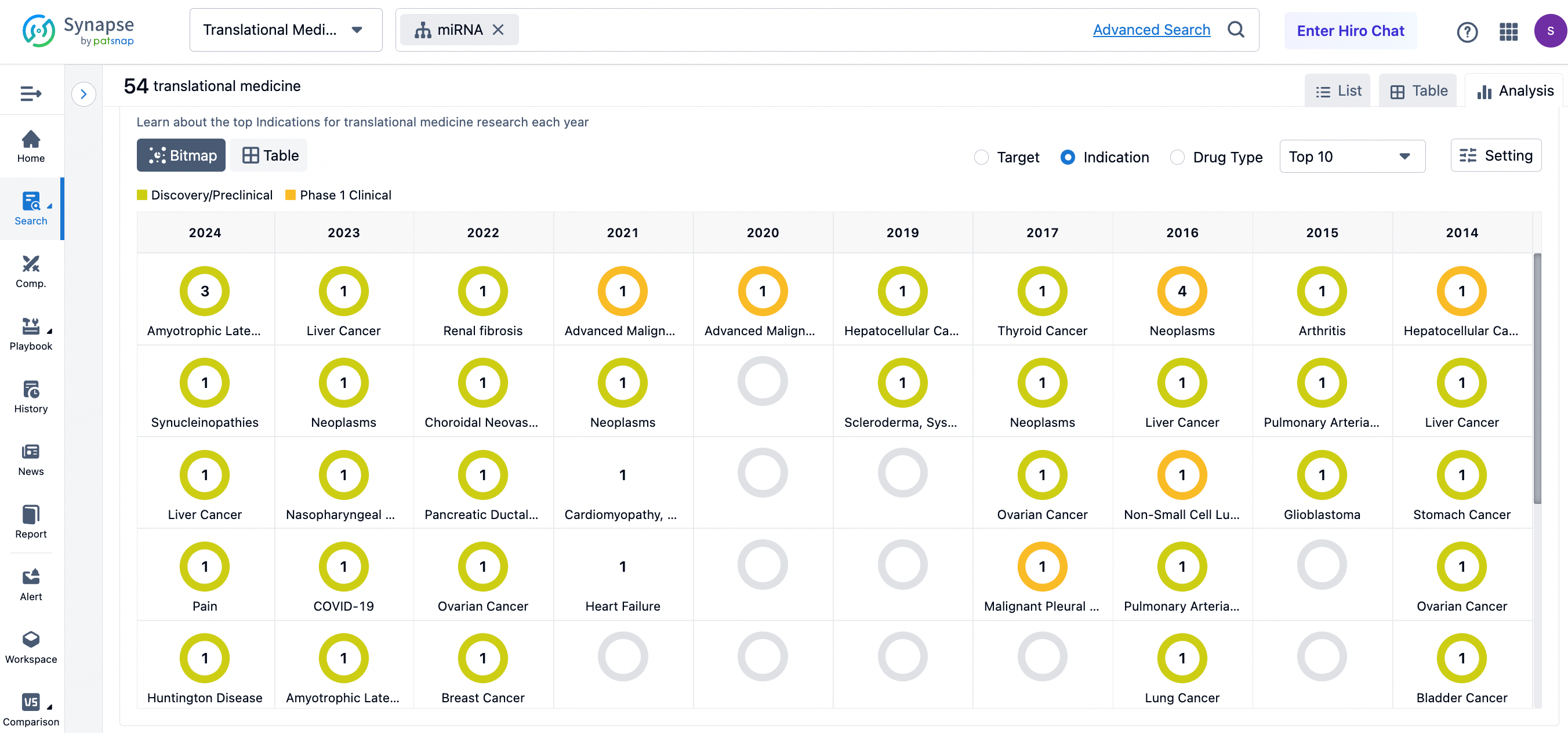
Recent queries in the Patsnap Synapse translational medicine research section have revealed a significant focus on numerous miRNA targets, with several drugs currently in the clinical translation stage. For instance, miR-486-5p is a miRNA that plays a role in non-malignant diseases, particularly active in liver cells and the immune system, and is abundant in muscle and human plasma. miR-155 is crucial in the immune system, particularly expressed in lymphocytes, where it plays a vital regulatory role in immune development and function. miR-128 is considered a potential biomarker and therapeutic target due to its possible involvement in various clinical processes. miR-203a-3p has shown promise in chronic pain treatment by specifically regulating pain sensitivity responses in the hypothalamic arcuate nucleus. miR-223-3p is associated with the side effects of pyrimidine-based drugs in hepatocellular carcinoma, indicating its potential role in early diagnosis and treatment of side effects in liver cancer. miR-186-5p plays a role in tumor angiogenesis by regulating pro-angiogenic pathways in endothelial cells, making it a potential target for anti-angiogenic cancer therapies. Additionally, miR-621 is viewed as a potential radiosensitizer due to its ability to enhance radiotherapy effects. Finally, the miR-17-92 cluster plays significant roles in various biological processes, including regulation of B-cell lymphoma and other immune cells, in contexts like the immune system and cardiovascular diseases. The expression and function of these miRNAs in different disease states reveal their tremendous potential as diagnostic markers and therapeutic targets.
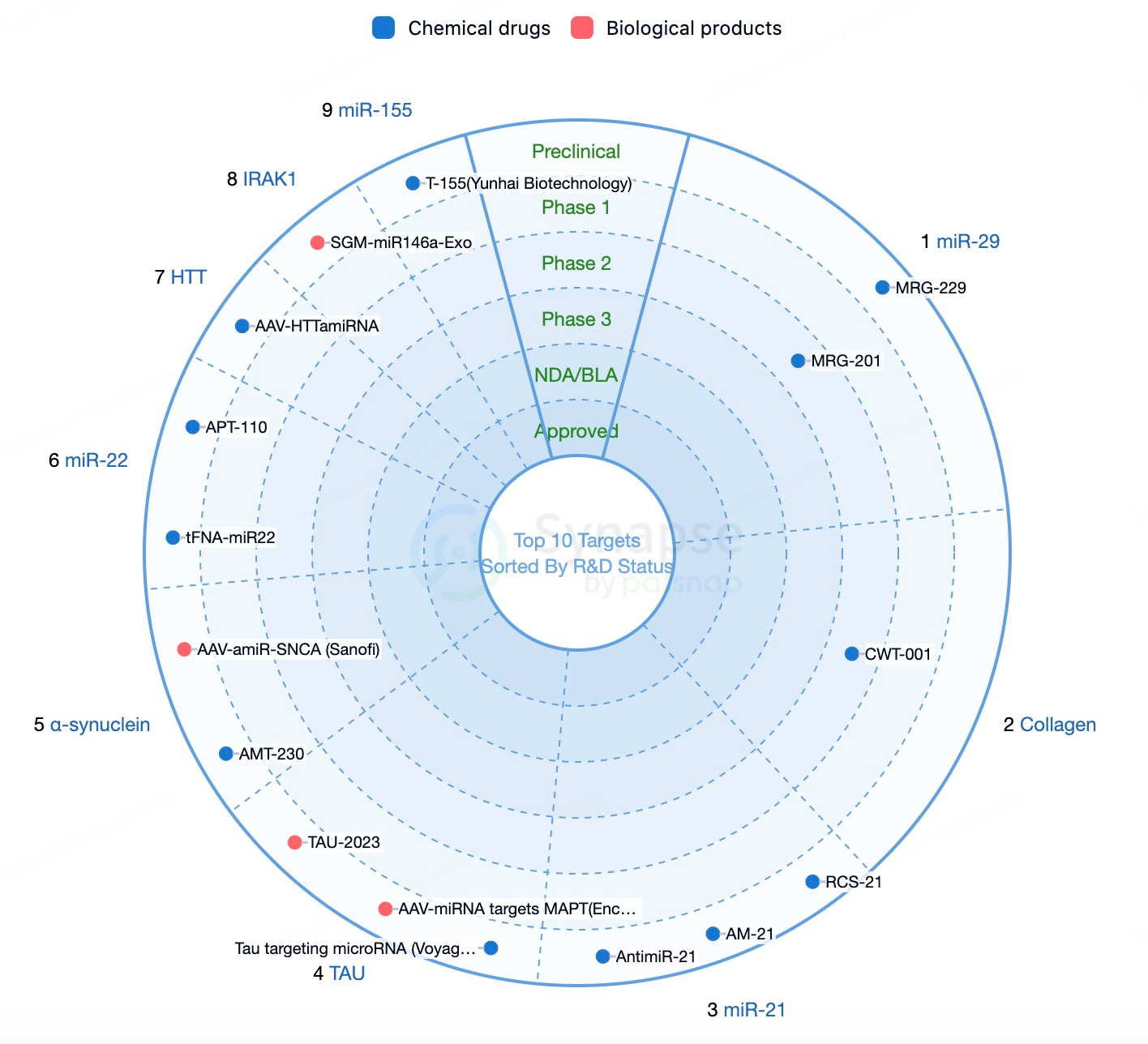
Global Competitive Landscape of miRNA Drug Development
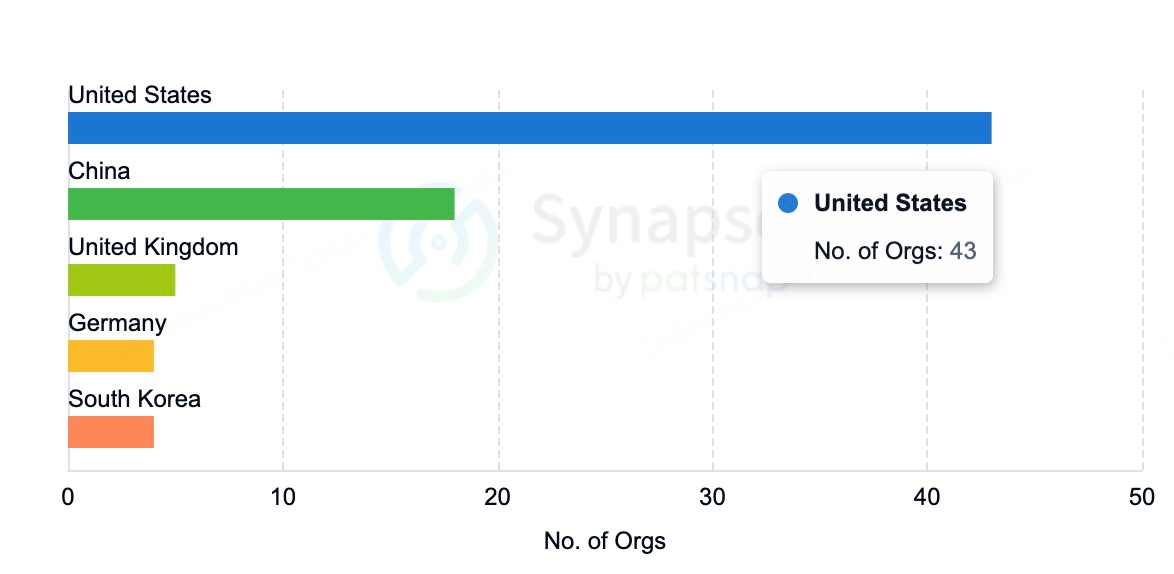
miRNA drug development activities are widely distributed globally, particularly in North America, Europe, and Asia. Alnylam Pharmaceuticals, based in the United States, leads the RNAi therapy field, with several RNAi drugs in late-stage clinical development. Alnylam is not only technologically advanced but has also established collaborations with other major pharmaceutical companies, further solidifying its leadership in the RNAi sector.
In China, companies such as Ribo Life Science, Sirnaomics Biopharmaceuticals, and GenePharma are playing increasingly important roles in miRNA drug development. Notably, Ribo Life Science has collaborated with Boehringer Ingelheim to jointly develop innovative small nucleic acid therapies for non-alcoholic or metabolic dysfunction-associated steatohepatitis (NASH/MASH). Sirnaomics Biopharmaceuticals has its own development pipeline in the small nucleic acid drug field, demonstrating its vitality and innovation in this domain. GenePharma is renowned for its expertise in siRNA chemical synthesis, providing a solid foundation for miRNA drug development. UniQure, a company with deep expertise in gene therapy, particularly in utilizing AAV delivery systems, also has multiple therapeutic pipelines in the miRNA space, with AMT-260 and AMT-130 having entered clinical phases I/II, showcasing its development strength in miRNA therapies.
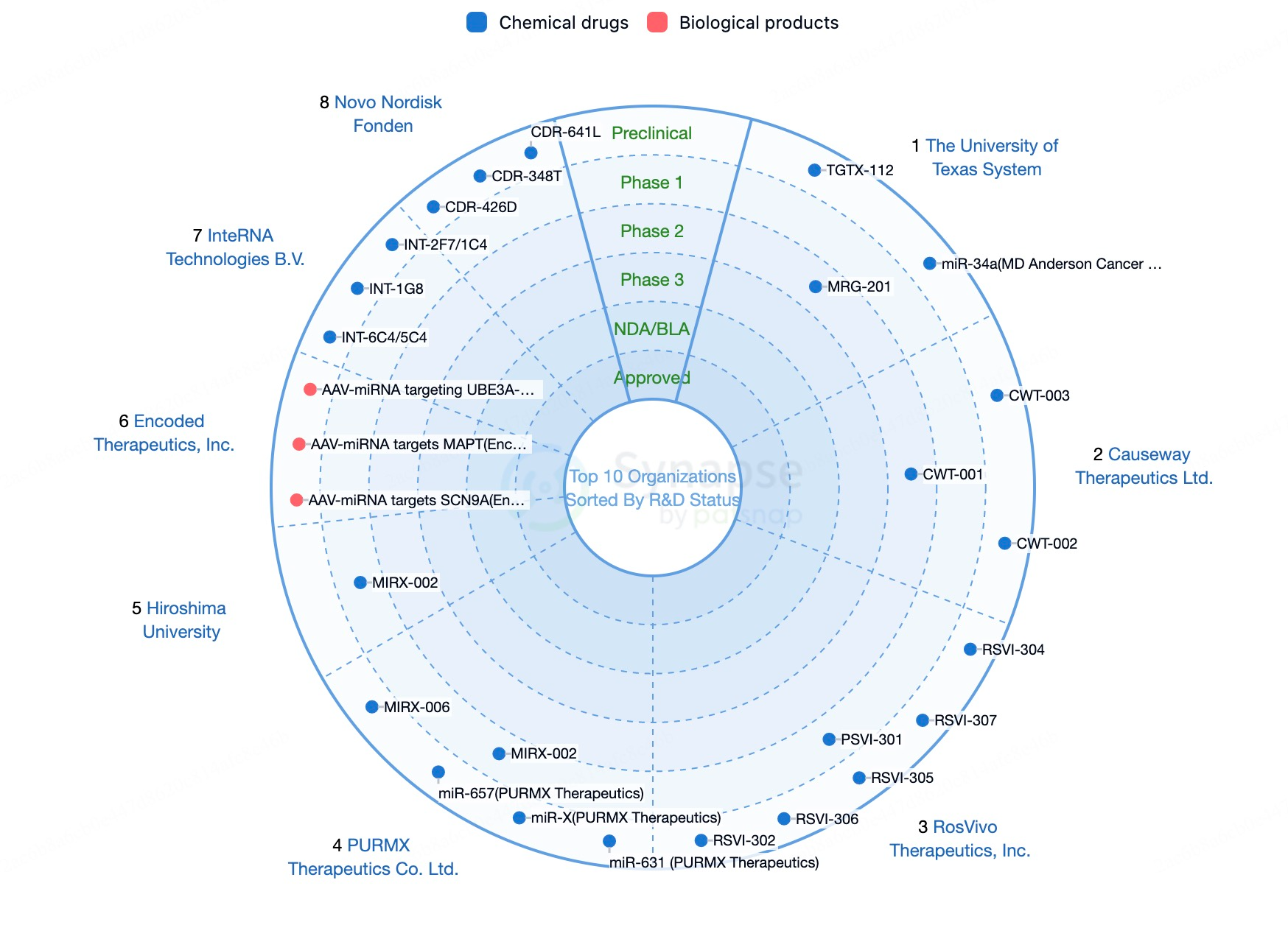
Looking ahead, the miRNA drug market is expected to continue growing with ongoing technological advancements and industry development. While no miRNA drug products are currently on the market, we can infer the future potential of the miRNA drug market by examining the development of mRNA technology. mRNA technology has demonstrated significant market potential in preventive vaccines, tumor immunotherapy, and other therapeutic areas, with projections estimating that the mRNA pharmaceuticals market could reach approximately $23 billion by 2035. Given the similarities between miRNA drugs and mRNA technology, both being RNA-based therapeutic approaches involved in regulating gene expression, it is reasonable to speculate that the miRNA drug market may also achieve rapid development in the coming years.
Challenges in the Application of miRNA Drugs
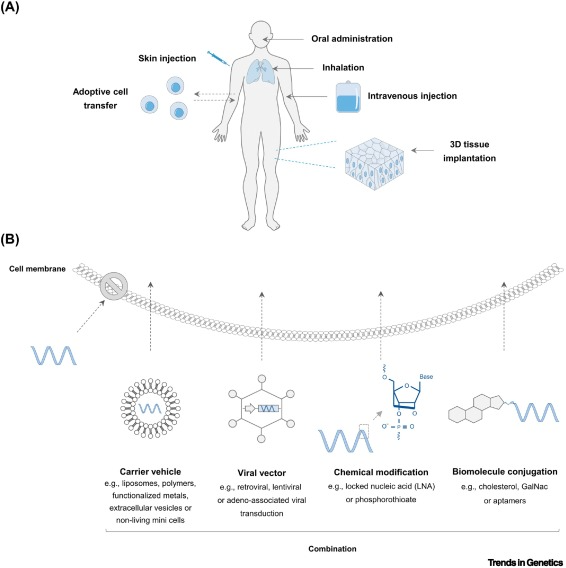
The technological challenges facing miRNA drugs primarily include target validation, optimization of delivery systems, and enhancement of stability. Target validation is a crucial step in determining whether a miRNA is suitable as a drug target, requiring the identification of specific miRNAs associated with particular diseases and clarifying their central role in disease progression. Optimizing delivery systems is essential to ensure that miRNAs effectively reach target tissues and release within cells, while improving stability is necessary to resist degradation by intracellular nucleases. Additionally, advancements in delivery technologies, particularly non-viral carriers like GalNac (N-acetylgalactosamine) and LNPs (lipid nanoparticles), are critical to the success of miRNA drugs. GalNac, as a novel delivery system, can enhance the delivery efficiency and targeting of miRNA drugs in vivo, while LNPs are favored for their excellent performance in protecting miRNAs from nuclease degradation. These technological advancements help overcome barriers related to the stability and targeted delivery of miRNA drugs, paving new pathways for their development. Moreover, chemical modifications of miRNA molecules can improve their stability and half-life in vivo, thereby enhancing their therapeutic efficacy. With ongoing advancements in delivery and chemical modification technologies, the development of miRNA drugs is moving toward safer and more effective solutions, bringing hope for their clinical application in the future.
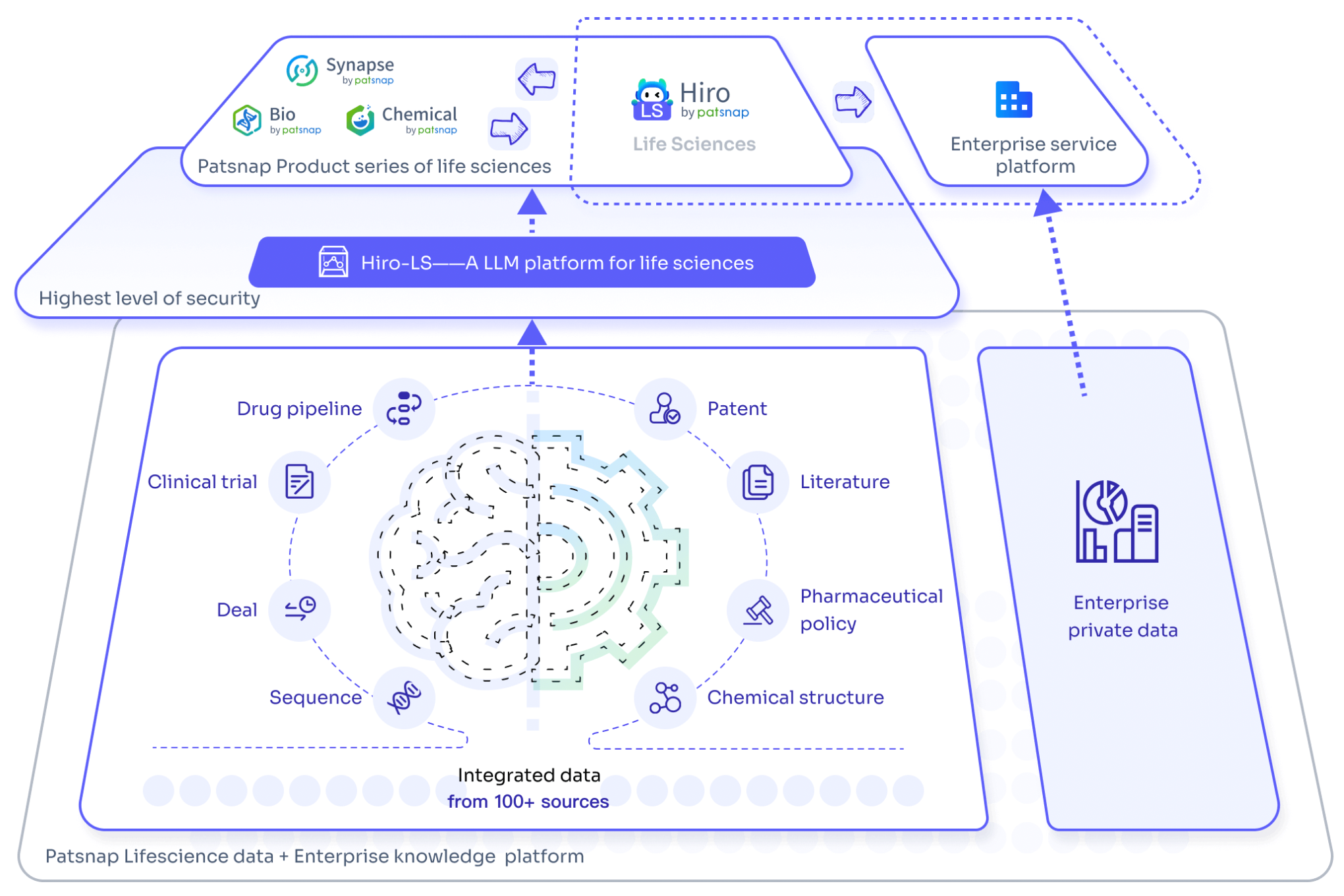
Hiro LS: Supporting miRNA Drug Development and Market Analysis
Through the biopharmaceutical AI model " Hiro LS", you can gain in-depth access to the latest insights in miRNA research and industry trends. Hiro LS not only efficiently integrates and analyzes the latest global scientific achievements but also utilizes advanced algorithmic models to predict potential drug targets and therapeutic mechanisms, summarizing the historical development and milestones of miRNA, helping researchers identify the most promising research directions at early stages and avoid inefficient resource allocation.
In terms of market competition analysis, Hiro LS can monitor global market dynamics for miRNA drugs in real-time, predict market capacity, and provide in-depth analyses of competitors' strategic layouts and technological advancements, offering customized competitive intelligence for enterprises. Through Hiro LS's intelligent analysis, companies can promptly adjust their R&D strategies and seize market opportunities. Importantly, Hiro LS provides an exceptionally smooth interactive Q&A experience, akin to having face-to-face discussions with seasoned industry experts, capable of addressing professional challenges while offering insightful advice. This intelligent support significantly enhances work efficiency and injects innovative thinking and strategic perspectives into research and business decisions. With comprehensive support from Hiro LS, enterprises and research institutions can not only keep pace with industry advancements but also lead future innovation directions, securing a competitive edge in a fierce market landscape.
For an experience with the large-scale biopharmaceutical model Hiro-LS, please click here for a quick and free trial of its features!
Conclusion
As a significant class of non-coding RNA molecules, miRNAs play a critical role in gene expression regulation, with their abnormal expression closely linked to the onset and progression of various diseases. Consequently, miRNA drugs are viewed as a promising therapeutic avenue, particularly suitable for targeting “undruggable” targets that traditional drugs struggle to reach. The mechanism of miRNA action primarily involves binding to the 3' untranslated region (3' UTR) of target mRNAs, inhibiting gene translation or promoting mRNA degradation; this multi-target regulatory approach is particularly important for treating complex diseases like cancer. By utilizing miRNA mimics or inhibitors, specific miRNAs can be supplemented or suppressed, offering new strategies for treating diseases such as cancer. Although no miRNA drugs have yet entered phase III clinical trials, the successful commercialization of siRNA drugs indicates the commercial value of RNA interference technology in drug development, suggesting a promising market outlook for miRNA drugs.
From a market perspective, miRNA drugs exhibit tremendous potential in disease prevention, diagnosis, and treatment, especially in areas like oncology, immune diseases, and metabolic disorders. Companies like Ribo Life Science, Sirnaomics Biopharmaceuticals, and GenePharma are actively participating in the development of miRNA drugs in China, showcasing the country’s rise in this field. However, the development of miRNA drugs still faces numerous challenges, including target validation, optimization of delivery systems, and drug stability. To overcome these challenges, researchers are exploring novel delivery technologies such as LNPs and GalNac, along with employing chemical modifications to enhance the stability of miRNAs. With continuous technological advancements and deeper clinical research, miRNA drugs are expected to achieve breakthrough progress in the coming years and ultimately be applied in clinical practice, providing new treatment options for patients.
Reference:
- 1.Rupaimoole, R. and F.J. Slack, MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov, 2017. 16(3): p. 203-222.
- 2.Fabian, M.R. and N. Sonenberg, The mechanics of miRNA-mediated gene silencing: a look under the hood of miRISC. Nat Struct Mol Biol, 2012. 19(6): p. 586-93.
- 3.Diener, C., A. Keller, and E. Meese, Emerging concepts of miRNA therapeutics: from cells to clinic. Trends Genet, 2022. 38(6): p. 613-626.