Monte Rosa Therapeutics and Novartis Ink Global Deal for VAV1-Targeted Therapies
Monte Rosa Therapeutics, Inc., a biotechnology firm in the clinical stage focused on creating innovative medicines based on molecular glue degraders (MGDs), has entered into a worldwide exclusive license agreement for development and commercialization with Novartis. This partnership aims to further the advancement of VAV1 MGDs, which includes MRT-6160.
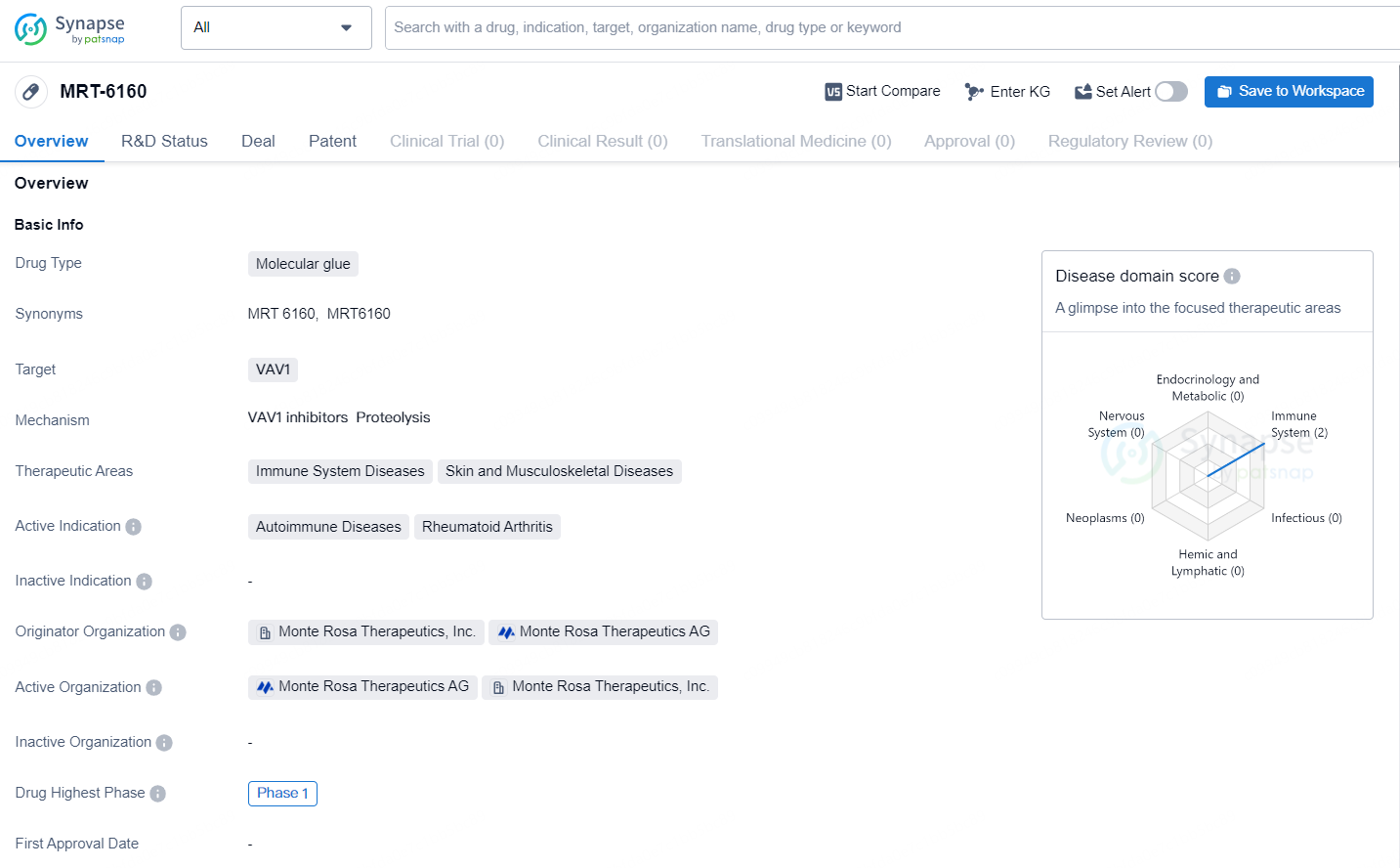
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
MRT-6160 is currently undergoing a Phase 1 study involving single ascending doses (SAD) and multiple ascending doses with healthy volunteers, focusing on immune-mediated disorders. According to the agreement, Novartis will acquire exclusive global rights to develop, produce, and market MRT-6160 along with other VAV1 molecular glue degraders (MGDs), and will take charge of all clinical development and commercialization efforts, commencing with Phase 2 clinical trials. Meanwhile, Monte Rosa will continue to manage the ongoing Phase 1 trials for MRT-6160.
"We are very pleased to share this partnership with Novartis, a significant entity in the field of immune-mediated disorders, and we are enthusiastic about the transformative opportunities this brings for both Monte Rosa and MRT-6160. We anticipate that this collaboration will expedite and expand the clinical development process for MRT-6160, enhancing this unique orally bioavailable approach while ensuring significant value for Monte Rosa," stated Markus Warmuth, M.D., CEO of Monte Rosa Therapeutics.
"Novartis has a longstanding commitment to molecular glue degraders, which potentially address difficult biological targets. We are enthusiastic about their use in the realm of immunology and the initial advancements we’ve observed from Monte Rosa, especially with MRT-6160. We look forward to progressing MRT-6160 further while exploring its potential to serve as a new therapeutic option for individuals affected by various immune-mediated disorders," commented Fiona Marshall, President of Biomedical Research at Novartis.
MRT-6160 is an investigational degrader of VAV1, which is a critical signaling protein involved in the pathways of T- and B-cell receptors. It is characterized by its strong potency, high selectivity, and oral bioavailability. Preclinical investigations have indicated substantial degradation of VAV1, leading to a marked reduction in cytokines associated with immune-mediated conditions, without adversely affecting other proteins. MRT-6160 has exhibited encouraging efficacy in preclinical models representing multiple immune-mediated disorders.
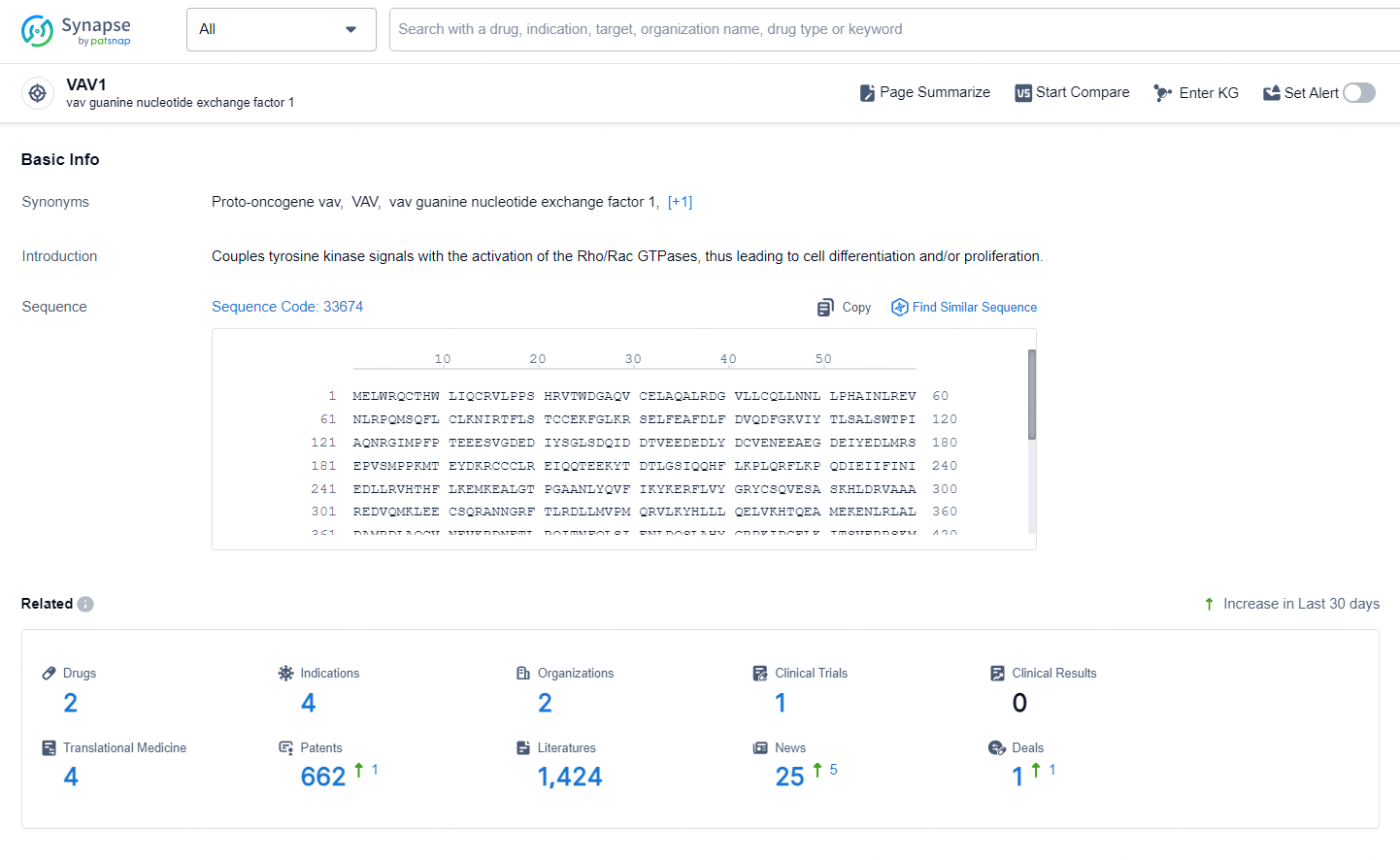
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of October 30, 2024, there are 2 investigational drugs for the VAV1 targets, including 4 indications, 2 R&D institutions involved, with related clinical trial reaching 1, and as many as 662 patents.
MRT-6160 represents a novel approach in drug development with its molecular glue mechanism of action and potential to address unmet medical needs in autoimmune diseases and rheumatoid arthritis. However, further research and development will be necessary to determine its clinical viability and eventual market potential.