Arrowhead Pharmaceuticals Seeks Trial Approval for Obesity Drug ARO-ALK7
Arrowhead Pharmaceuticals, Inc. (NASDAQ: ARWR) has disclosed that it has submitted a request for regulatory authorization to commence a Phase 1/2a clinical study of ARO-ALK7. This represents the company’s second RNA interference (RNAi) therapeutic candidate aimed at obesity treatment. Notably, ARO-ALK7 is the pioneering RNAi therapy designed to specifically target a gene found in adipose tissue. This development exemplifies Arrowhead’s expertise in delivering siRNA to various tissues and cell types throughout the body, utilizing its innovative and proprietary Targeted RNAi Molecule (TRiM™) platform.
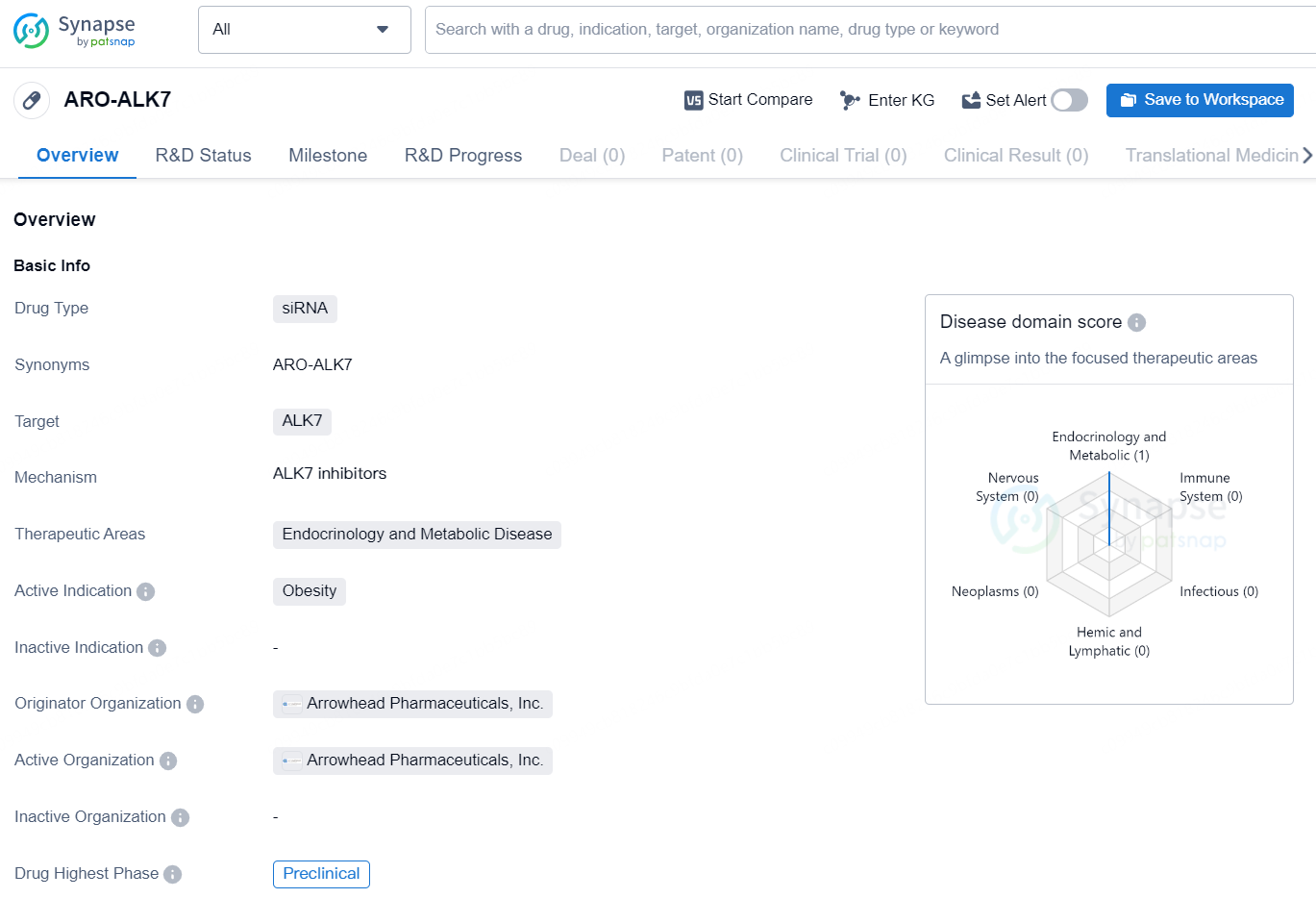
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Arrowhead currently has two RNAi-based candidates in clinical trials, ARO-ALK7 and ARO-INHBE, aimed at treating obesity. Both candidates employ innovative mechanisms, with ARO-INHBE targeting a ligand and ARO-ALK7 focusing on a receptor, to affect a pathway involved in fat storage within adipose tissue. Human genetic studies support both targets, indicating that individuals with loss-of-function mutations display more favorable body composition and metabolic profiles than those without these mutations, as noted by James Hamilton, M.D., Chief of Discovery and Translational Medicine at Arrowhead. He added, “The forthcoming Phase 1/2a trials will assess both single and multiple ascending doses of these candidates as monotherapies in obese individuals, as well as their efficacy when combined with incretin therapy for those with or without type 2 diabetes. We are optimistic that ARO-INHBE and ARO-ALK7 may effectively address existing gaps in current treatment standards and align with Arrowhead's focused efforts on RNAi-based therapies in the cardiometabolic field."
ARO-ALK7 specifically targets the silencing of the ACVR1C gene in adipocytes to limit the expression of Activin receptor-like kinase 7 (ALK7), which serves as a receptor in the energy homeostasis pathway associated with adipose tissue. Analysis of extensive genetic datasets indicates that lower ACVR1C levels correlate with improved adipose distribution and a decreased likelihood of experiencing obesity-related metabolic issues. The exploratory treatment of ARO-ALK7 holds promise for diminishing visceral fat and enhancing lipid and glycemic metrics.
An application has been submitted to the New Zealand Medicines and Medical Devices Safety Authority for the approval of the clinical trial, which will be reviewed by the Standing Committee on Therapeutic Trials. Once authorized, Arrowhead plans to launch AROALK7-1001, a Phase 1/2a first-in-human dose-escalation study aimed at determining the safety, tolerability, pharmacokinetics, and pharmacodynamics of ARO-ALK7 in as many as 90 adult participants with obesity. The initial phase of the trial will focus on the evaluation of ARO-ALK7 as a monotherapy, while the second phase will examine its use in combination with tirzepatide, a GLP-1/GIP receptor co-agonist, which received approvals in the U.S. and the European Union for managing type 2 diabetes mellitus in 2022 and for weight management in 2023/2024, respectively.
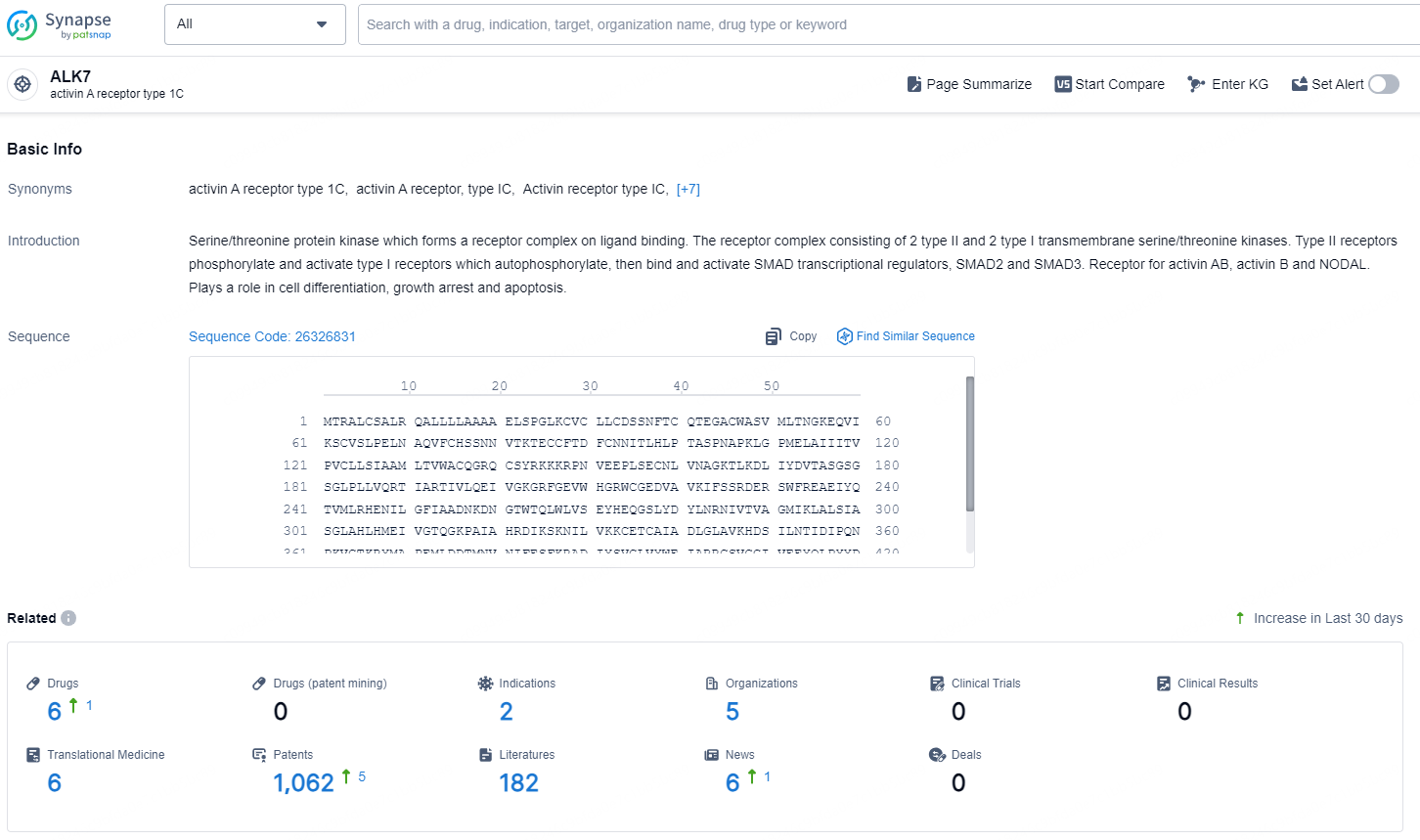
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of December 6, 2024, there are 6 investigational drugs for the ALK7 target, including 2 indications, 5 R&D institutions involved, and as many as 1062 patents.
ARO-ALK7 is a siRNA drug developed by Arrowhead Pharmaceuticals, Inc. The drug targets the ALK7 gene and is indicated for the treatment of obesity in the therapeutic areas of endocrinology and metabolic diseases. As of the latest information, ARO-ALK7 is in the preclinical phase of development, which is the earliest stage in the drug development process.