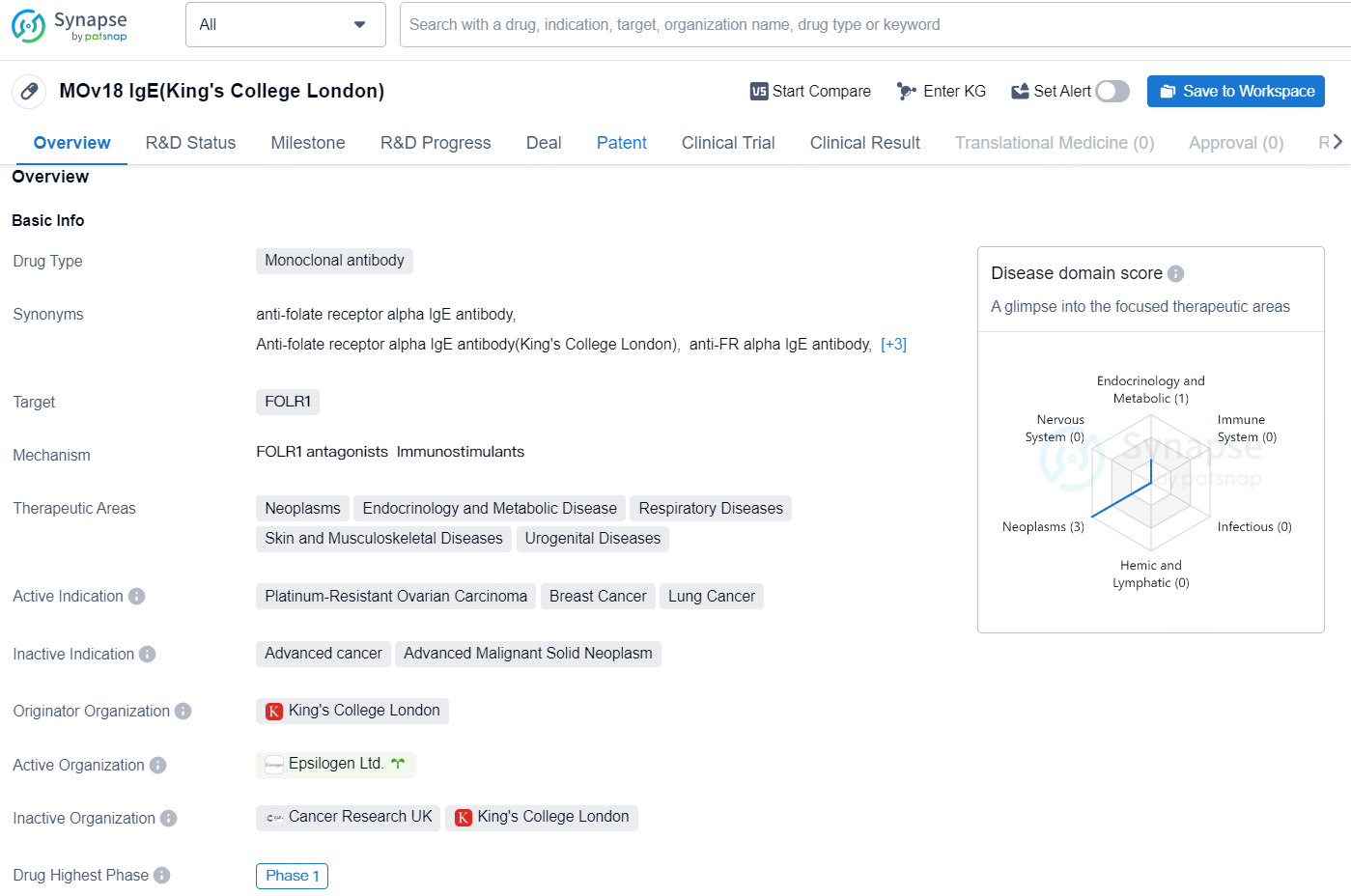
Epsilogen has launched a Phase Ib trial for MOv18 IgE
Epsilogen, a worldwide frontrunner in the advancement of immunoglobulin E (IgE) antibodies for cancer treatment, has declared the initiation of a Phase Ib trial assessing MOv18 IgE in individuals suffering from platinum-resistant ovarian cancer (PROC).
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The Phase Ib, open-label trial focused on dose escalation and expansion (NCT06547840) is set to recruit 45 patients diagnosed with PROC, whose condition has worsened after undergoing a maximum of four prior treatment regimens. This study aims to evaluate the safety, tolerability, and effectiveness of MOv18 IgE within increasing dose groups.
MOv18 IgE is an IgE monoclonal antibody that specifically targets the folate receptor alpha (FR alpha) antigen, which is predominantly found in several cancers, such as ovarian, non-small cell lung, endometrial, and triple-negative breast cancer. It marks the inaugural entry of an IgE antibody therapeutic into clinical trials, with this Phase Ib study expected to produce substantial translational data to enhance the understanding of the distinct mechanism of action of IgE in humans. Previous results from a Phase I safety assessment of MOv18 IgE indicated that it was well tolerated and safe, with indications of anti-tumor efficacy reported in Nature Communications.
Dr. Tim Wilson, CEO of Epsilogen, expressed: “We are delighted to commence the Phase Ib study of MOv18 IgE, following promising Phase I results. This represents a significant advancement in our mission to provide this innovative therapeutic approach to cancer patients.”
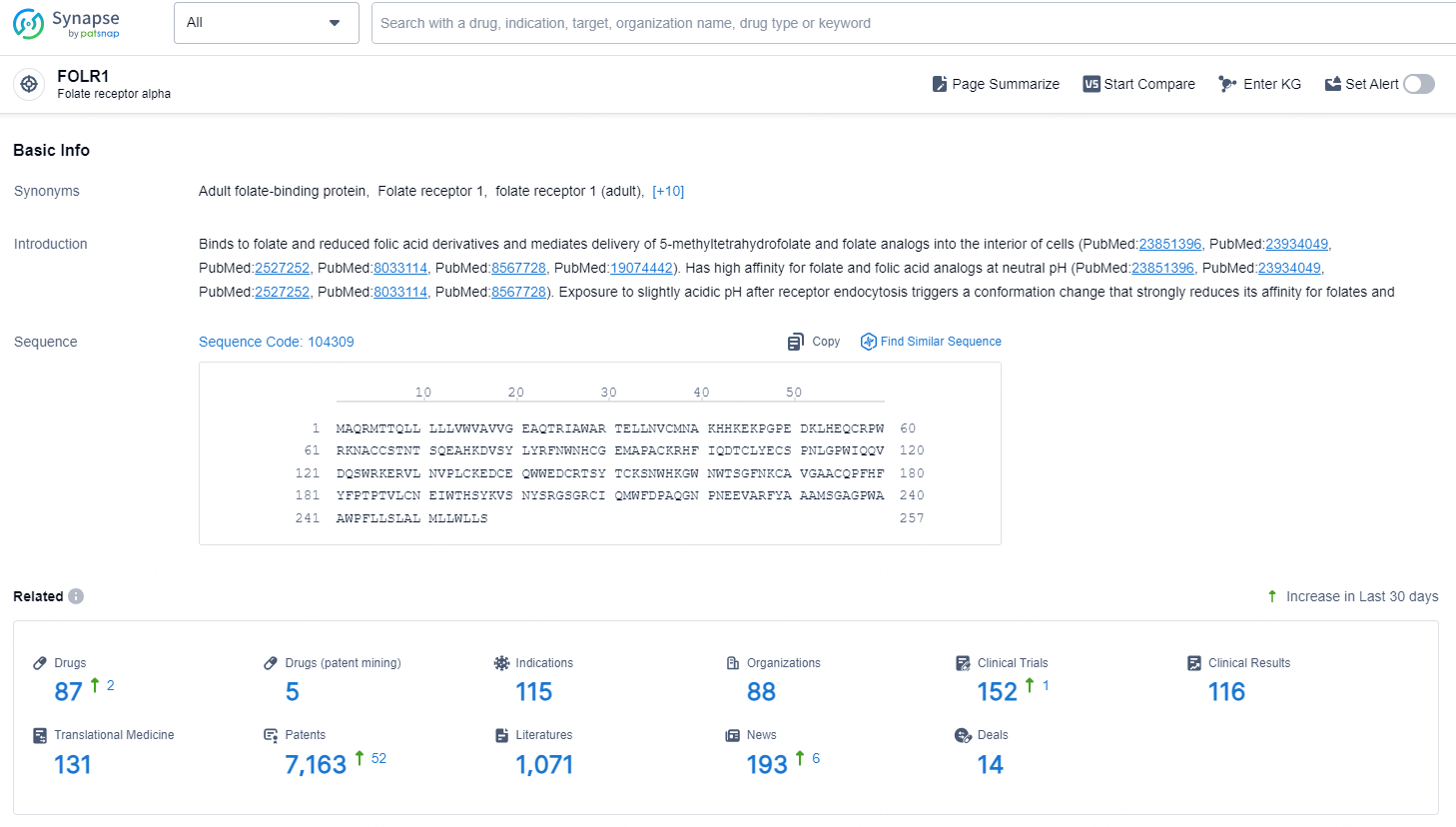
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Chemical, As of December 6, 2024, there are 87 investigational drugs for the FOLR1 target, including 115 indications, 88 R&D institutions involved, with related clinical trials reaching 152, and as many as 7163 patents.
The drug MOv18 IgE(King's College London) is a monoclonal antibody that targets FOLR1 and is being developed for the treatment of various therapeutic areas, including neoplasms, endocrinology and metabolic diseases, respiratory diseases, skin and musculoskeletal diseases, and urogenital diseases.