TORL BioTherapeutics Appoints Aran Maree as CMO and Launches Phase 2 Trial for Ovarian Cancer Treatment
TORL BioTherapeutics, LLC (TORL), a biotechnology firm at the clinical stage focused on discovering and creating innovative antibody-based immunotherapies aimed at enhancing and prolonging the lives of cancer patients globally, has declared the appointment of Aran Maree, MD, to the position of Chief Medical Officer. Furthermore, TORL has launched CATALINA-2, a worldwide Phase 2 trial for its unique Claudin 6 (CLDN6) targeted antibody-drug conjugate (ADC) TORL-1-23 in individuals with CLDN6 positive (CLDN6+) platinum-resistant ovarian cancer (PROC).
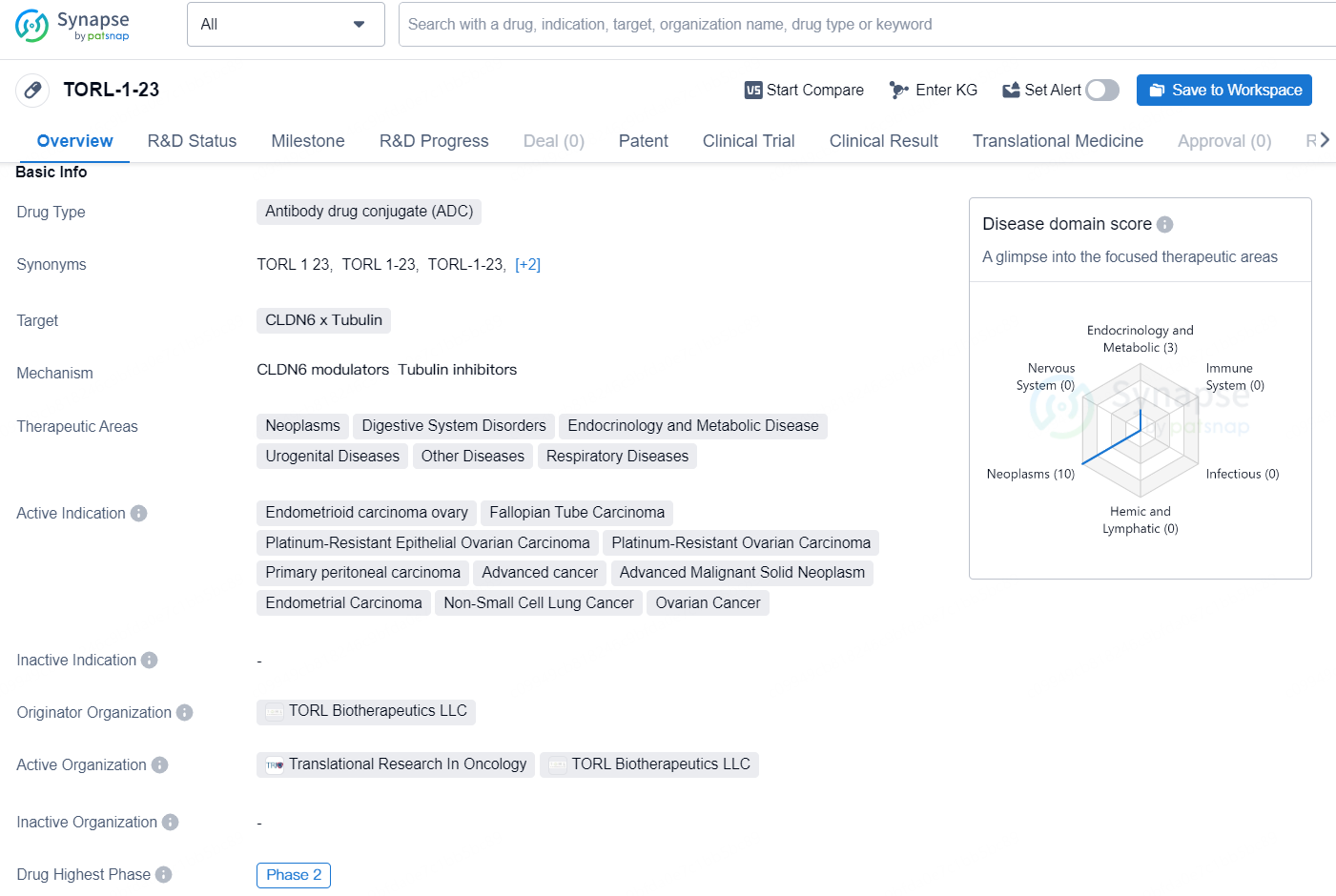
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"2024 marks a pivotal year for TORL, as we embark on our inaugural Phase 2 trial with the Claudin 6 targeted antibody-drug conjugate TORL-1-23 while simultaneously progressing several antibody-focused clinical initiatives," stated Mark J. Alles, Chairman and CEO of TORL Biotherapeutics. "Aran Maree brings exceptional expertise and experience as a physician-leader in the biopharmaceutical field, and his extensive history of enhancing patient outcomes across various diseases will be invaluable to TORL."
Dr. Maree most recently held the position of Chief Medical Officer at Johnson & Johnson (J&J) Innovative Medicines, previously known as Janssen. In this capacity, he led a dedicated team focusing on the clinical and scientific development of established products, patient safety, and pediatric advancements while co-chairing the R&D Development Committee, which evaluated clinical programs across different therapeutic areas, including oncology, immunology, neurology, cardiovascular, metabolic, and infectious diseases. His contributions to safety oversight and program development have influenced some of the most notable products in J&J's history, along with numerous significant drugs currently in development.
Dr. Maree joined J&J in 2006 and progressively took on medical roles within the MedTech division across Australia, New Zealand, Asia Pacific, and Japan. In these roles, he developed a robust medical affairs structure while enhancing regulatory, quality, and health outcomes/access functions. In 2012, he was elevated to global Chief Medical Officer for J&J MedTech, overseeing practices for patient monitoring and safety surveillance, promoting clinical data transparency, and ensuring scientific integrity at the convergence of biology and technology. He later became Chief Medical Officer of the pharmaceutical division in 2017.
Before his tenure at J&J, Dr. Maree was with The Boston Consulting Group and Merck & Co. (MSD) in Australia and New Zealand. He earned his honors medical degree from the Royal College of Surgeons in Ireland/National University of Ireland. Dr. Maree trained as a physician in Dublin and obtained his Membership of the Royal College of Physicians of Ireland (MRCPI), and further specialized in interventional cardiology in both Dublin, Ireland, and Sydney, Australia.
"The potential of TORL-1-23 and the Company's expanding pipeline of new target programs present an extraordinary chance to advance clinical practices in oncology," remarked Aran Maree, MD, Chief Medical Officer. "I am excited to join the TORL team at such a crucial juncture and am looking forward to building and expanding a global medical organization that aligns with the needs of our portfolio and the patients we aim to serve."
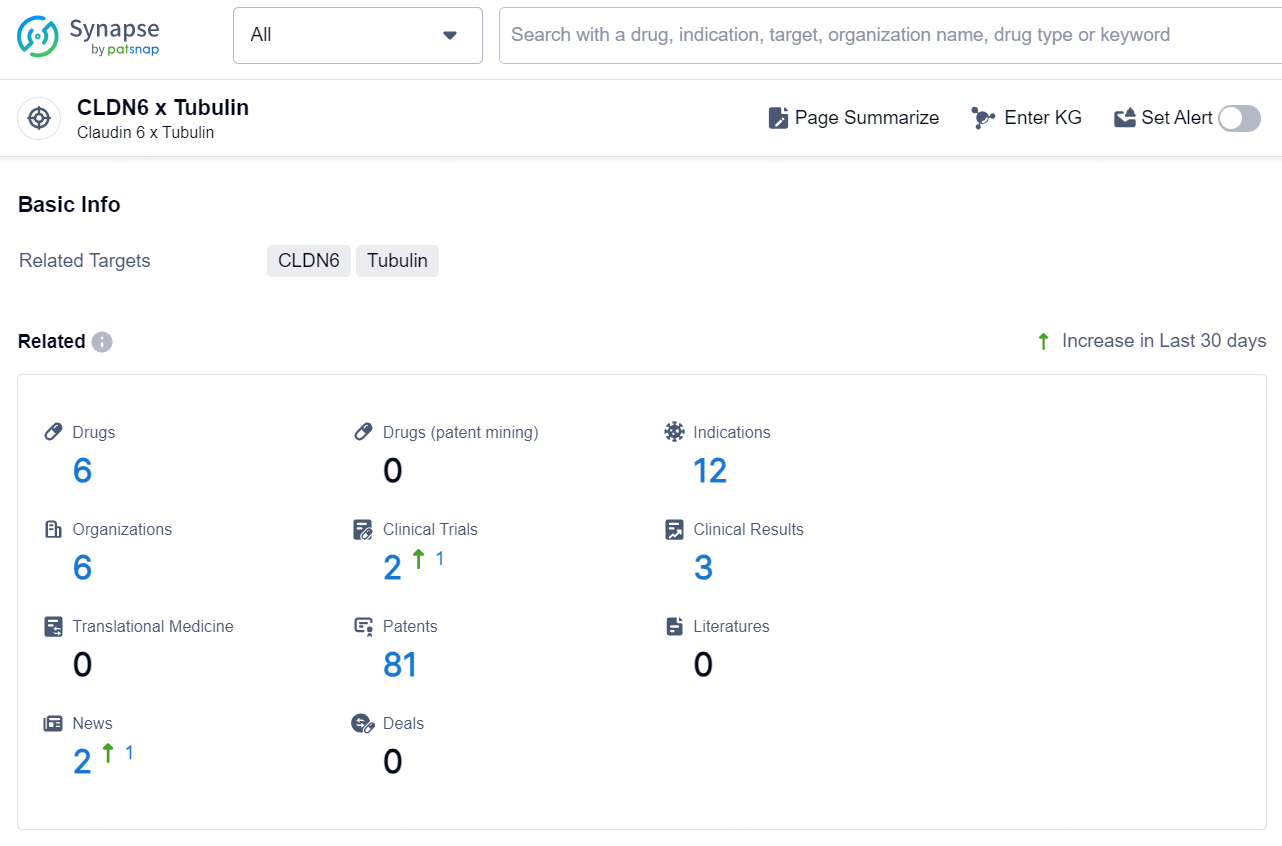
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of December 6, 2024, there are 6 investigational drugs for the CLDN6 x Tubulin target, including 12 indications, 6 R&D institutions involved, with related clinical trials reaching 2, and as many as 81 patents.
TORL-1-23 is an antibody drug conjugate (ADC) that targets CLDN6 x Tubulin and is being developed by TORL Biotherapeutics LLC. The drug is primarily focused on the treatment of a variety of neoplastic disorders and associated conditions such as digestive system disorders, endocrinology and metabolic diseases, urogenital diseases, respiratory diseases and other diseases.