Jasper Therapeutics Begins Phase 1b/2a ETESIAN Trial for Briquilimab in Asthma
Jasper Therapeutics, Inc. (Nasdaq: JSPR) is a biotechnology firm in the clinical phase concentrating on the advancement of briquilimab, an innovative antibody treatment targeting c-Kit (CD117) for conditions driven by mast cells, including chronic spontaneous urticaria (CSU), chronic inducible urticaria (CIndU), and asthma. The company has recently reported that the initial patient has received treatment in its Phase 1b/2a clinical challenge study, named ETESIAN (Evaluating The Efficacy and Safety of briquilimab In participANts with allergic asthma), which assesses briquilimab's effectiveness in allergic asthma. The ETESIAN trial is focused on a single subcutaneous administration of briquilimab in asthma patients.
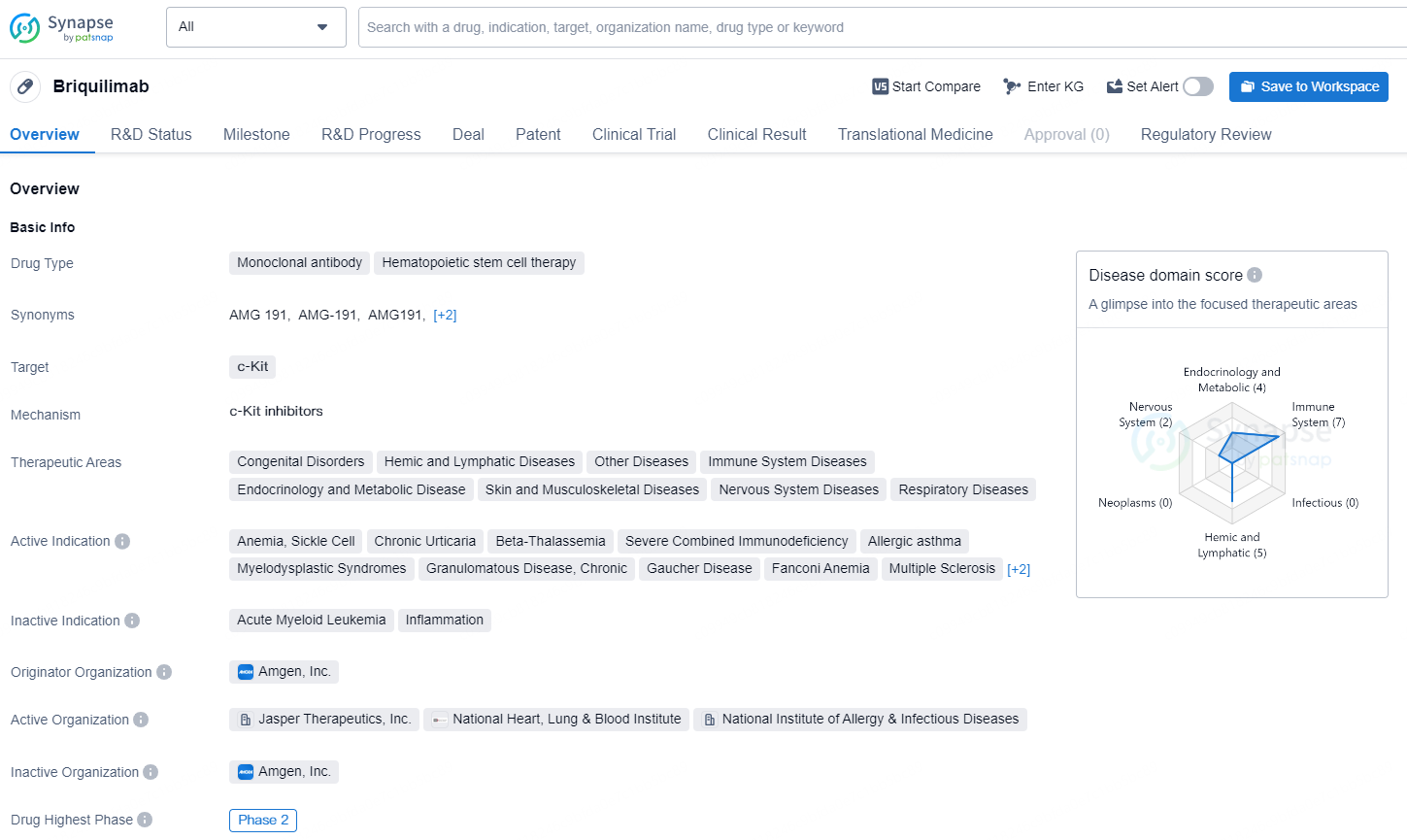
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"The initiation of dosing for the first participant in our ETESIAN trial targeting asthma represents a crucial achievement, constituting our third clinical initiative assessing briquilimab in diseases characterized by excessive mast cell activity," stated Edwin Tucker, M.D., Chief Medical Officer of Jasper. “After successfully escalating doses during Part 2 of the BEACON study in chronic spontaneous urticaria (CSU), we received regulatory approval to immediately administer a subcutaneous dose of 180mg in the ETESIAN study. We believe this approach will significantly reduce mast cell levels in the airways and lead to lasting clinical improvements for asthma patients. We are eager to share updates regarding enrollment as the study advances and look forward to presenting initial findings in the latter half of 2025.”
The Phase 1b/2a ETESIAN trial is designed as a single-dose, double-blind, placebo-controlled challenge study that aims to enroll around 30 participants at up to 7 sites throughout Canada. A primary goal of this trial is to establish proof-of-concept for asthma treatment at a proposed therapeutic dose, guiding future research in broader asthma populations. The study will utilize a single subcutaneous administration of 180mg briquilimab, with key evaluations focusing on both early and late asthmatic responses, alterations in airway hyperresponsiveness, mast cell depletion and recovery, as well as safety profiles.
"Mast cell reduction through c-Kit inhibition introduces an innovative mechanism that could significantly reduce asthmatic responses in patients who lack sufficient options with current therapies," noted Paul O’Byrne, M.D., Professor, Dean, and Vice President of the Faculty of Health Sciences at McMaster University. "Given its role as a robust and targeted c-Kit inhibitor, I am optimistic that briquilimab can address the safety concerns associated with other c-Kit inhibiting drugs, thus becoming an essential treatment alternative for those with asthma. I am looking forward to the patient enrollment process for the ETESIAN study."
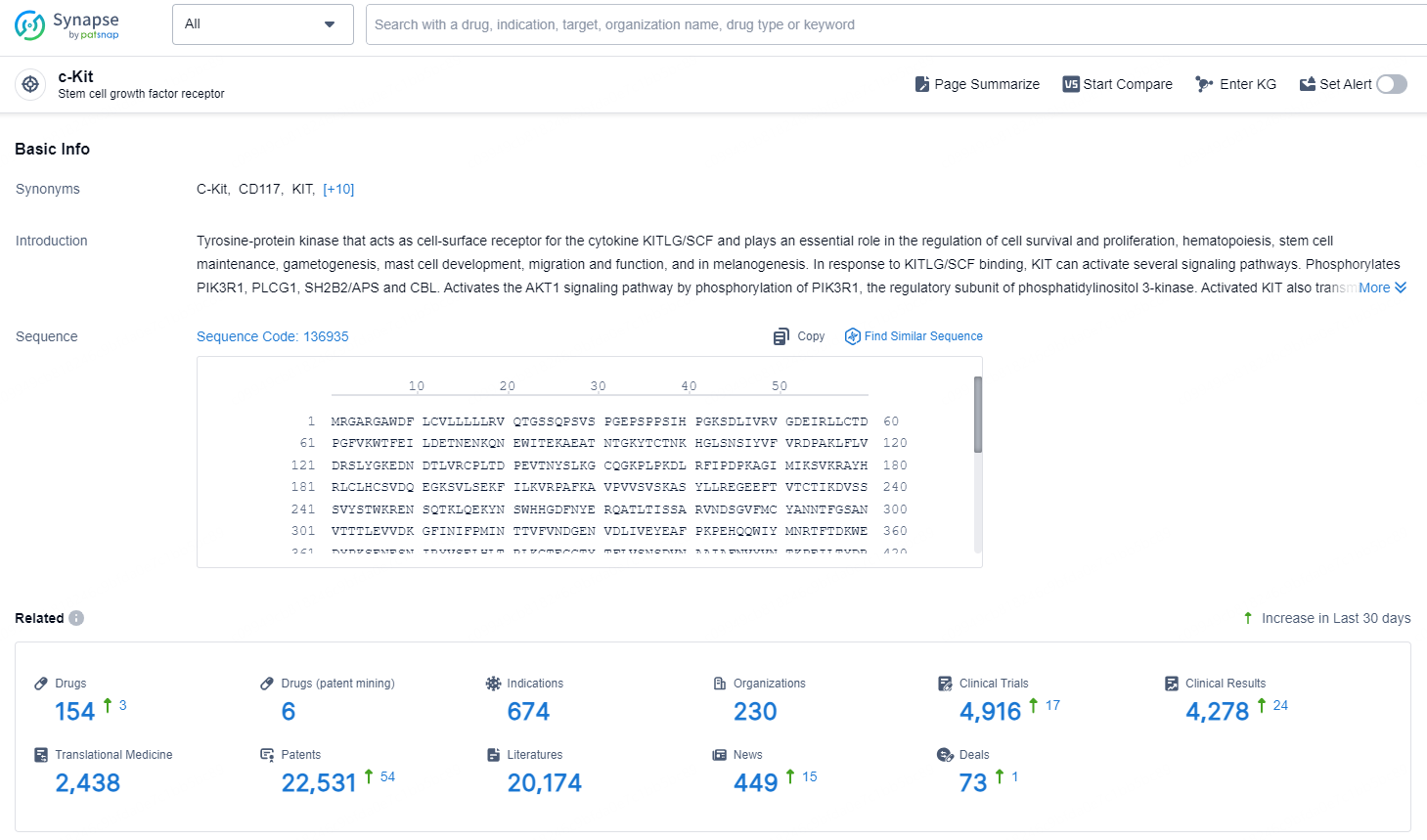
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Chemical, As of December 6, 2024, there are 154 investigational drugs for the c-Kit target, including 674 indications, 230 R&D institutions involved, with related clinical trials reaching 4916, and as many as 22531 patents.
Briquilimab is a monoclonal antibody and hematopoietic stem cell therapy that targets the c-Kit protein. It has a wide range of therapeutic areas, including congenital disorders, hemic and lymphatic diseases, immune system diseases, endocrinology and metabolic diseases, skin and musculoskeletal diseases, nervous system diseases, and respiratory diseases.