FDA Approves OncoC4's IND for AI-081, a Novel PD-1/VEGF Therapy for Solid Tumors
OncoC4, Inc., a biopharmaceutical firm in the later phases of development focused on innovative treatments for cancer and neurological disorders, has disclosed that the U.S. Food and Drug Administration (FDA) has approved an investigational new drug (IND) application for AI-081. This drug is a potentially leading PD-1 and VEGF bispecific antibody aimed at treating advanced solid tumors. After receiving the Study May Proceed notification from the FDA, OncoC4 plans to launch the BIPAVE-001 Phase 1/2 trial for advanced solid tumors in the first quarter of 2025.
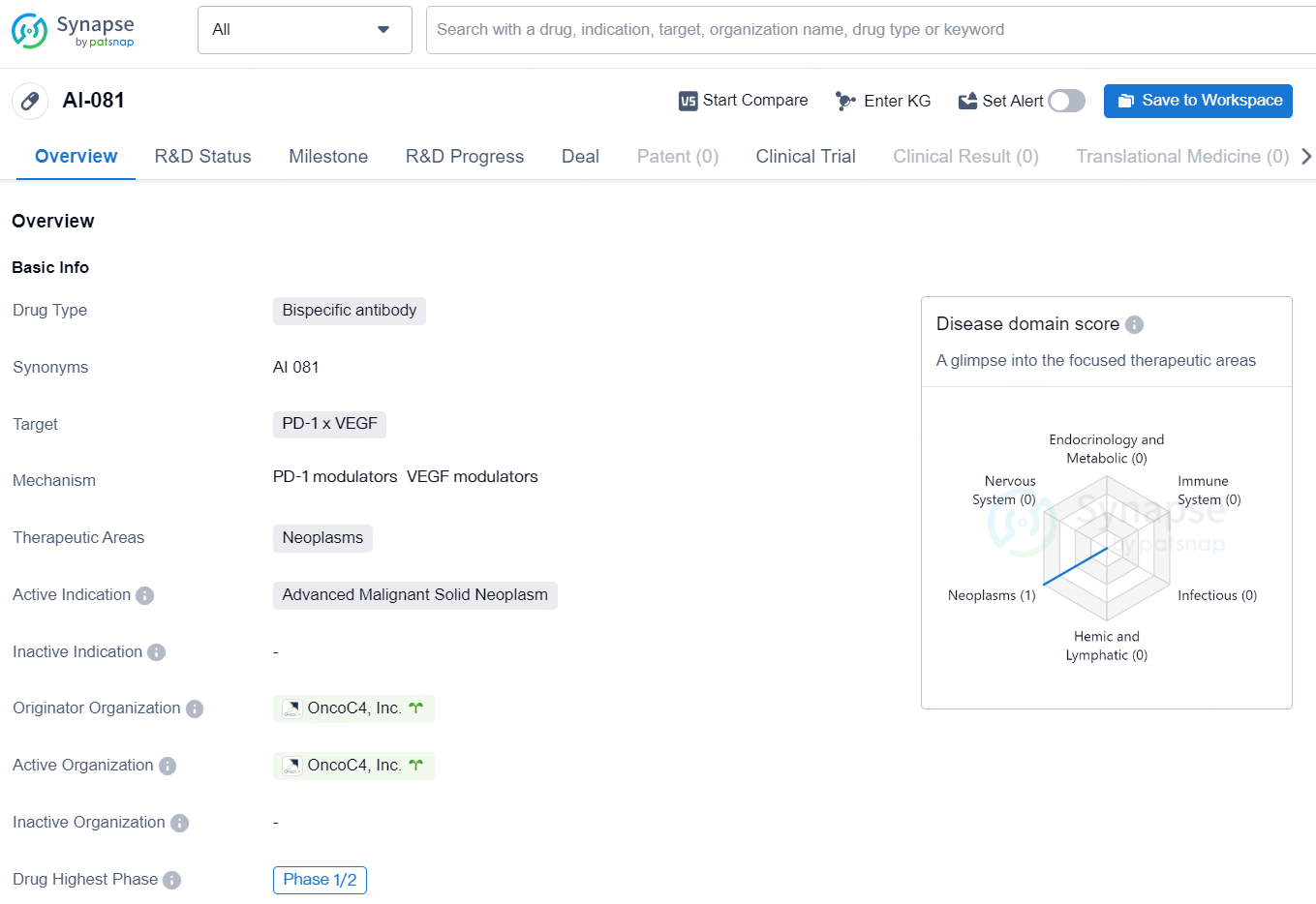
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"AI-081 utilizes two proprietary clinical-stage antibodies with high affinity for anti-PD1 and anti-VEGF, aimed at broadening the scope of checkpoint therapy," stated Yang Liu, PhD, Co-Founder, CEO, and Chief Scientific Officer (CSO) of OncoC4. "The optimal cooperative interactions of AI-081 have shown enhanced PD-1 inhibition in the presence of VEGF, resulting in strong anti-tumor efficacy across various preclinical assessments. We are eager to commence the clinical development of AI-081, with plans to dose the first patient in our BIPAVE-001 Phase 1/2 trial in early 2025."
BIPAVE-001 is a Phase 1/2 clinical trial aimed at assessing the safety, pharmacokinetics, and effectiveness of AI-081. The initial segment, Part A, will involve a dose escalation study as the first application in humans to establish the recommended Phase 2 dosage for AI-081 as a monotherapy in patients with advanced solid tumors.
AI-081 consists of proprietary high-affinity anti-PD1 (AI-025) and anti-VEGF (AI-011) antibodies. Designed with a tested head-and-tail bispecific approach, AI-081 seeks to enhance cooperative interactions, incorporating additional Fc silencing mutations to maintain immune effector cells. In preclinical studies—molecular, cellular, and in vivo-conducted to support the IND application, AI-081 exhibited superior affinity for both targets, significantly improved cooperative interactions, and enhanced in vivo effects compared to other candidates within the same class in various mouse models. Together, these results indicate that AI-081 has the potential to be a best-in-class therapeutic option, characterized by high affinity and cooperative interactions that foster strong, synergistic anti-tumor effects, especially when combined with PD-1 blockade in the presence of VEGF.
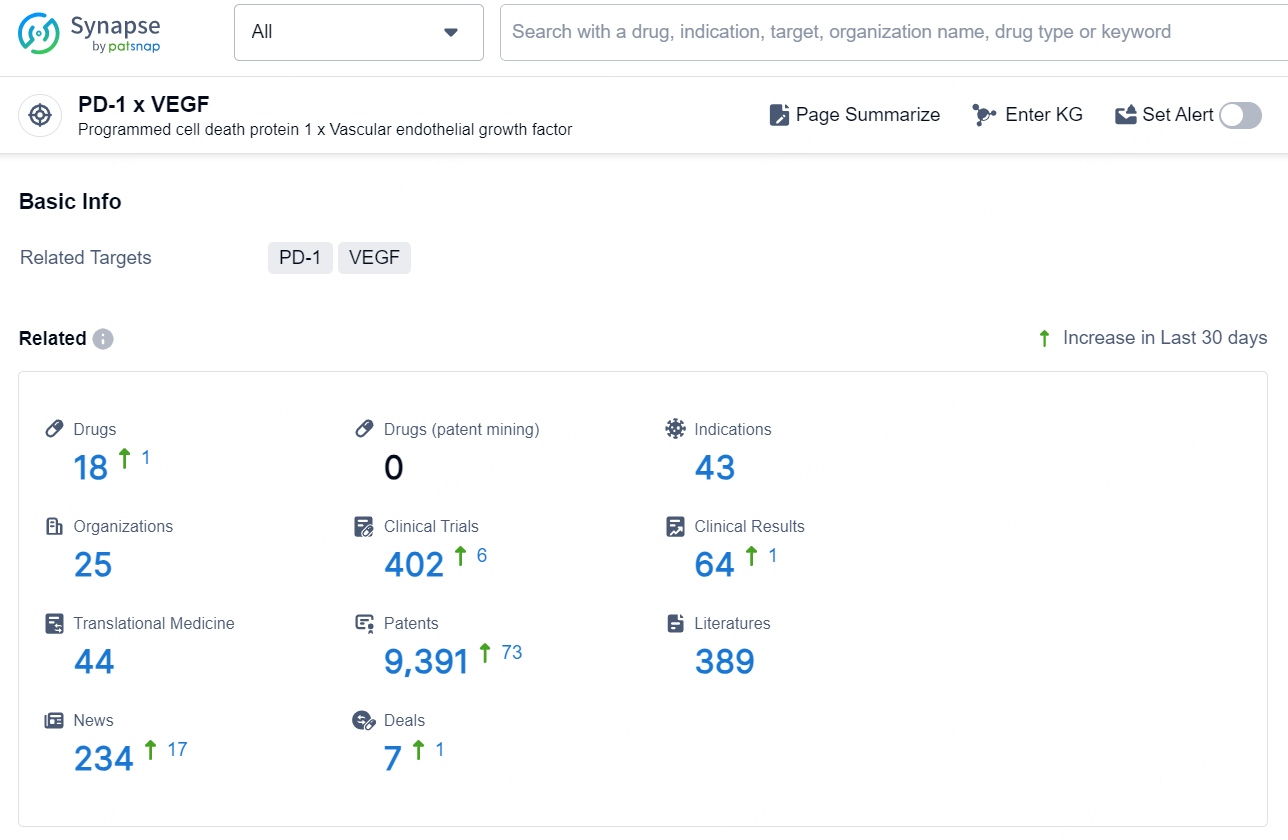
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of December 6, 2024, there are 18 investigational drug for the PD-1 x VEGF targets, including 43 indications, 25 R&D institutions involved, with related clinical trials reaching 402, and as many as 9391 patents.
AI-081 is a bispecific antibody drug developed by OncoC4, Inc. The drug targets the PD-1 and VEGF proteins and is intended for the treatment of neoplasms, specifically advanced malignant solid neoplasms. As of now, AI-081 is in Phase 1/2 of clinical trials, which suggests that it is still undergoing testing to evaluate its safety and efficacy in treating the targeted conditions.