Astria Therapeutics Gains FDA Approval for IND on Atopic Dermatitis Antibody STAR-0310
Astria Therapeutics, Inc. (Nasdaq:ATXS), a biopharmaceutical firm dedicated to creating transformative therapies for allergic and immunologic conditions, announced that it has received clearance from the U.S. Food and Drug Administration (FDA) for its Investigational New Drug (IND) application concerning STAR-0310. This monoclonal antibody serves as an OX40 antagonist and is being developed as a potential therapeutic option for atopic dermatitis (AD) and possibly other conditions. A Phase 1a trial involving healthy participants is set to begin in the first quarter of 2025, with initial proof-of-concept findings anticipated in the third quarter of 2025. Additionally, the company expects to obtain proof-of-concept results in patients with atopic dermatitis by the second quarter of 2026.
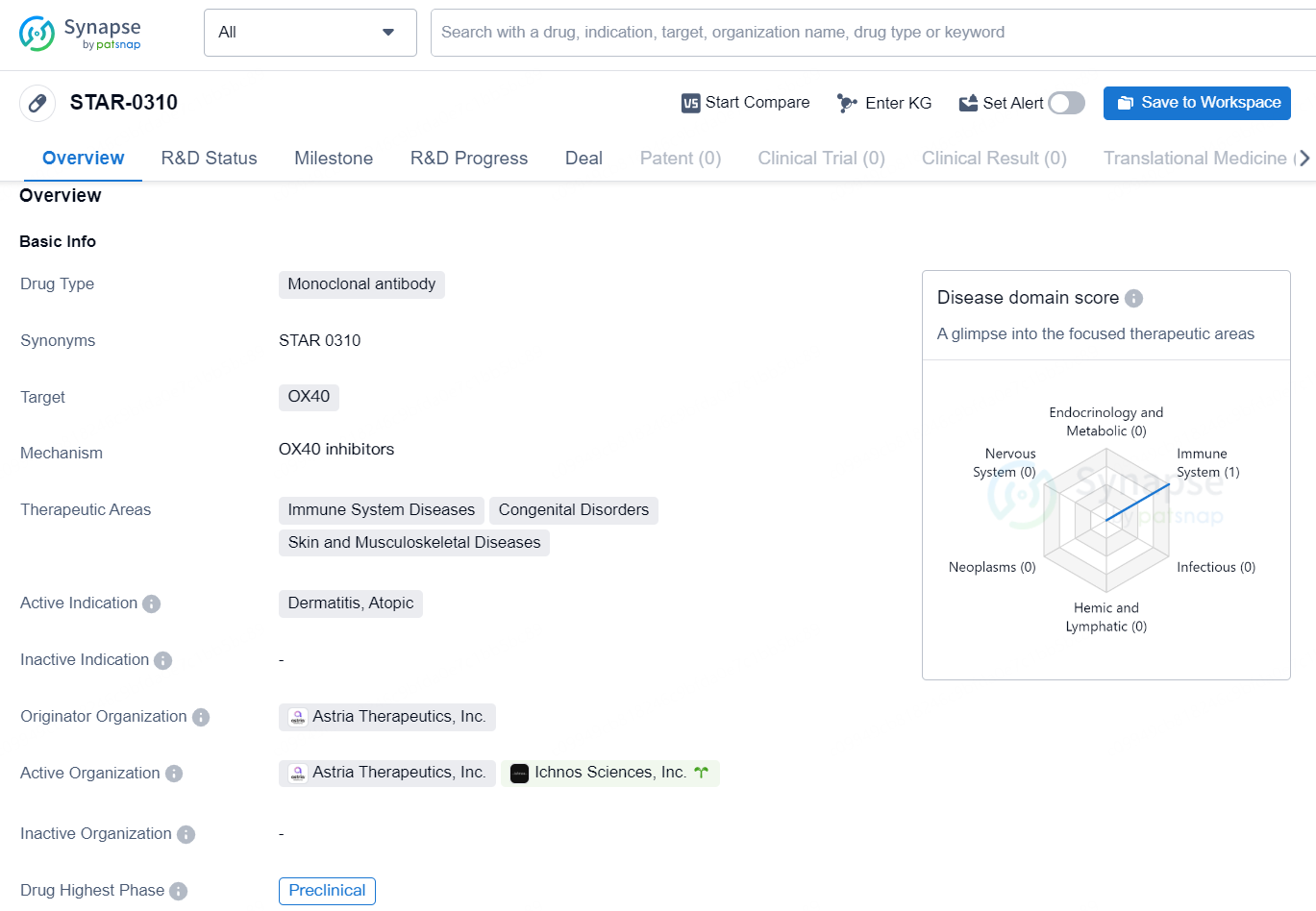
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
“We are very encouraged by the FDA’s approval of our IND application for STAR-0310, as it marks a significant advancement for the program,” stated Chris Morabito, M.D., Chief Medical Officer at Astria Therapeutics. “We are enthusiastic about the possibilities offered by the OX40 mechanism. Aiming to develop the leading OX40 program, we have purposefully crafted STAR-0310 to build upon insights gained from previous OX40 receptor and OX40 ligand initiatives. The high affinity and potency of STAR-0310, along with its low ADCC, may provide a broader therapeutic window. Furthermore, STAR-0310 may potentially be administered as infrequently as once every six months, thanks to its extended half-life and possible effects on disease modification.”
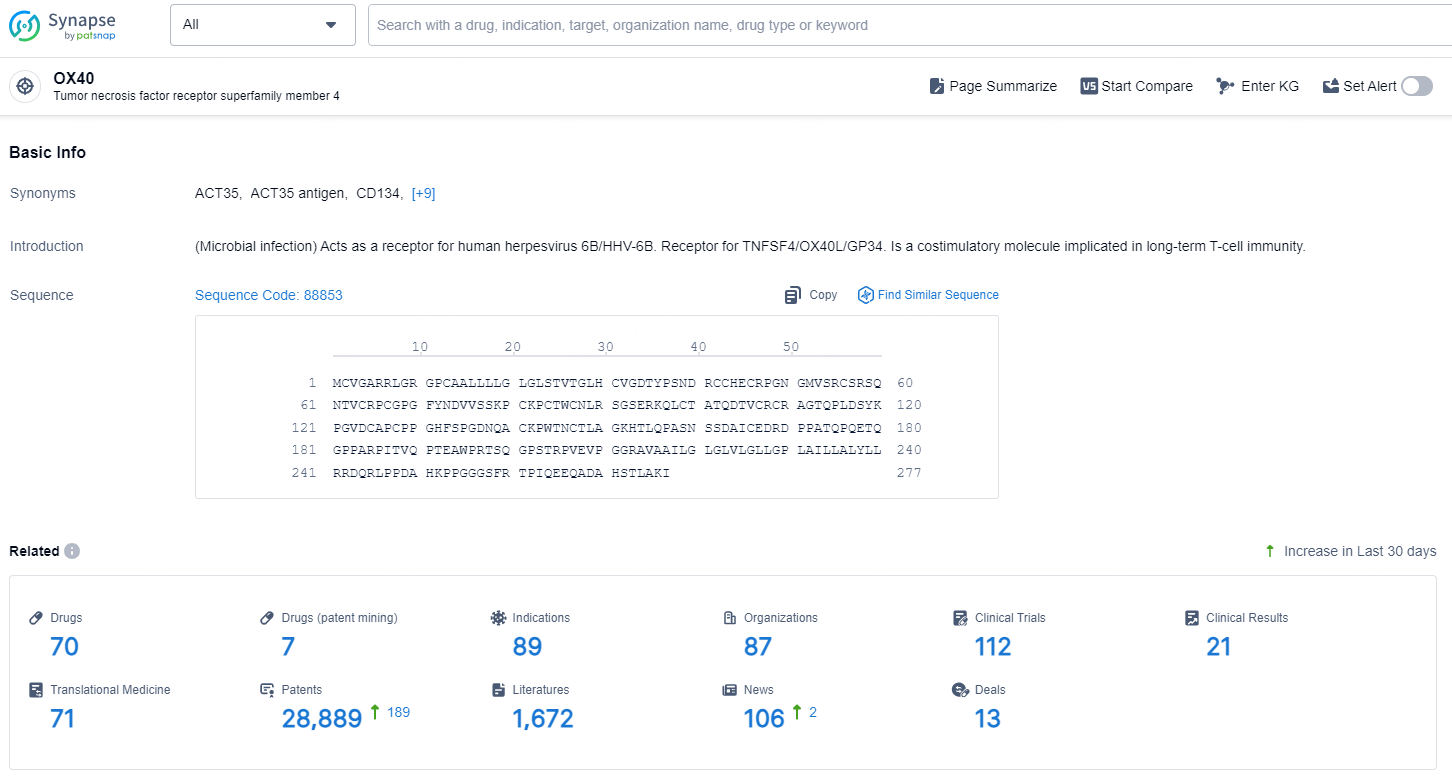
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of December 17, 2024, there are 70 investigational drugs for the OX40 targets, including 89 indications, 87 R&D institutions involved, with related clinical trials reaching 112, and as many as 28889 patents.
STAR-0310 is a monoclonal antibody drug developed by Astria Therapeutics, Inc. The drug targets OX40 and is being developed for the treatment of Dermatitis, Atopic, and other immune system diseases, congenital disorders, as well as skin and musculoskeletal diseases. As of the latest available information, STAR-0310 is in the preclinical phase, indicating that it is still in the early stages of development and has not yet progressed to clinical trials.