Barzolvolimab Meets Primary and Secondary Endpoints in Phase 2 Trial for Chronic Inducible Urticaria
Celldex Therapeutics, Inc. reported favorable outcomes from its Phase 2 clinical study of barzolvolimab targeting two prevalent types of chronic inducible urticaria (CIndU)—cold urticaria and symptomatic dermographism.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The research involves patients who continue to experience symptoms after antihistamine treatment. Barzolvolimab is a humanized monoclonal antibody that selectively attaches to the receptor tyrosine kinase KIT, effectively blocking its function, which is crucial for the operation and longevity of mast cells.
Chronic Inducible Urticaria (CIndU) is marked by the development of hives or wheals in response to identifiable triggers, such as exposure to cold in ColdU and skin manipulation in Scratching Dermatitis (SD). The activation of mast cells is recognized as a key factor in both ColdU and SD.
Dr. Jonathan Bernstein presented this data in a late-breaking oral session at the Annual Scientific Meeting of the American College of Allergy, Asthma & Immunology in Boston. He is a Professor of Clinical Medicine at the University of Cincinnati Medical Center and a Partner at the Bernstein Allergy Group and Clinical Research Center.
“Barzolvolimab is the first medication to demonstrate efficacy in a large, randomized, placebo-controlled trial for chronic inducible urticaria. We are thrilled to announce that all primary and secondary endpoints of the study were statistically significant and clinically relevant. We are committed to advancing this potential new treatment for patients and anticipate beginning Phase 3 trials for inducible urticaria in 2025,” stated Diane C. Young, MD, Senior Vice President and Chief Medical Officer at Celldex Therapeutics.
Barzolvolimab functions as a humanized monoclonal antibody that interacts with the receptor tyrosine kinase KIT with high specificity, effectively inhibiting its function. KIT is present in various cell types, including mast cells, which are responsible for mediating inflammatory reactions, such as allergies and hypersensitivity. The signaling through KIT governs the development, migration, survival, and functionality of mast cells. In certain inflammatory conditions like chronic urticaria, the activation of mast cells significantly contributes to the initiation and worsening of the disease.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of October 30, 2024, there are 144 investigational drugs for the c-Kit target, including 648 indication, 218 R&D institutions involved, with related clinical trial reaching 4916, and as many as 2050 patents.
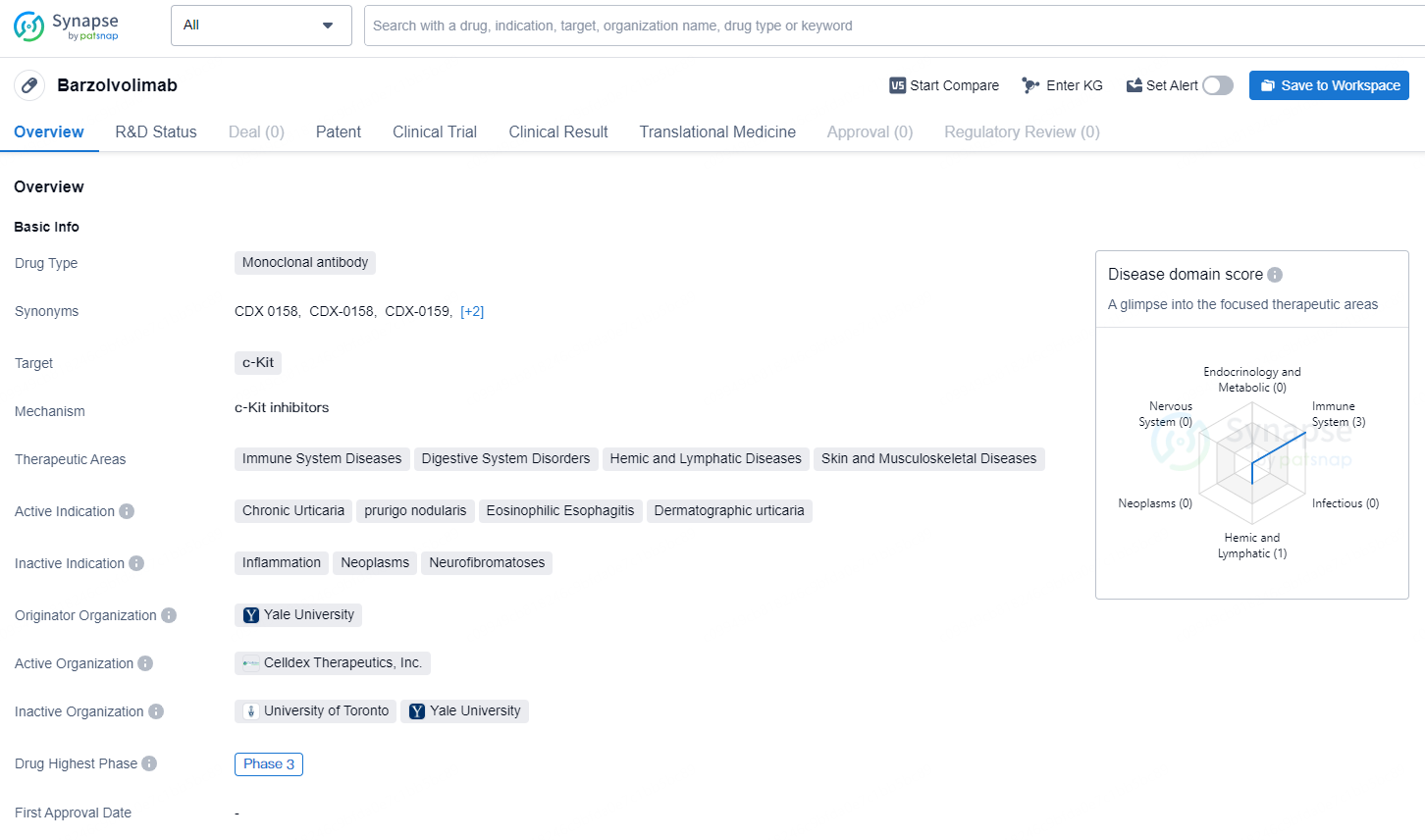
Barzolvolimab is a monoclonal antibody drug that targets c-Kit, and it has a therapeutic focus on immune system diseases, digestive system disorders, hemic and lymphatic diseases, as well as skin and musculoskeletal diseases. The drug is currently being developed for the treatment of chronic urticaria, prurigo nodularis, eosinophilic esophagitis, and dermatographic urticaria.