Bivalent Ligands for GPCRs
Ortho ligand and allo ligand
The ligands of GPCRs can be divided into orthosteric and allosteric ligands based on the location of their binding sites. Orthosteric ligands bind at the classical endogenous ligand binding site, while allosteric ligands include all allosteric binding sites that are distinct from the orthosteric ones.
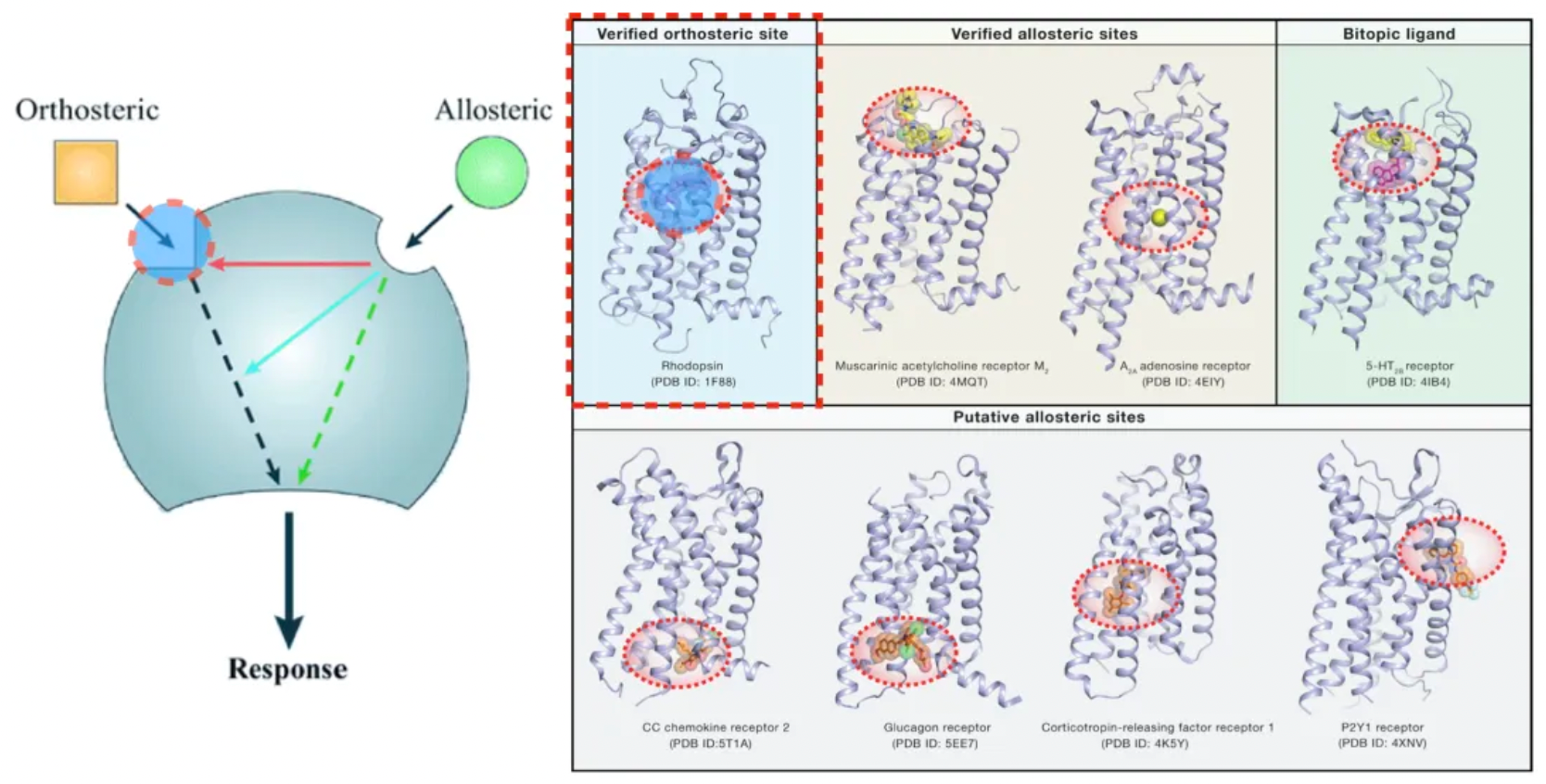
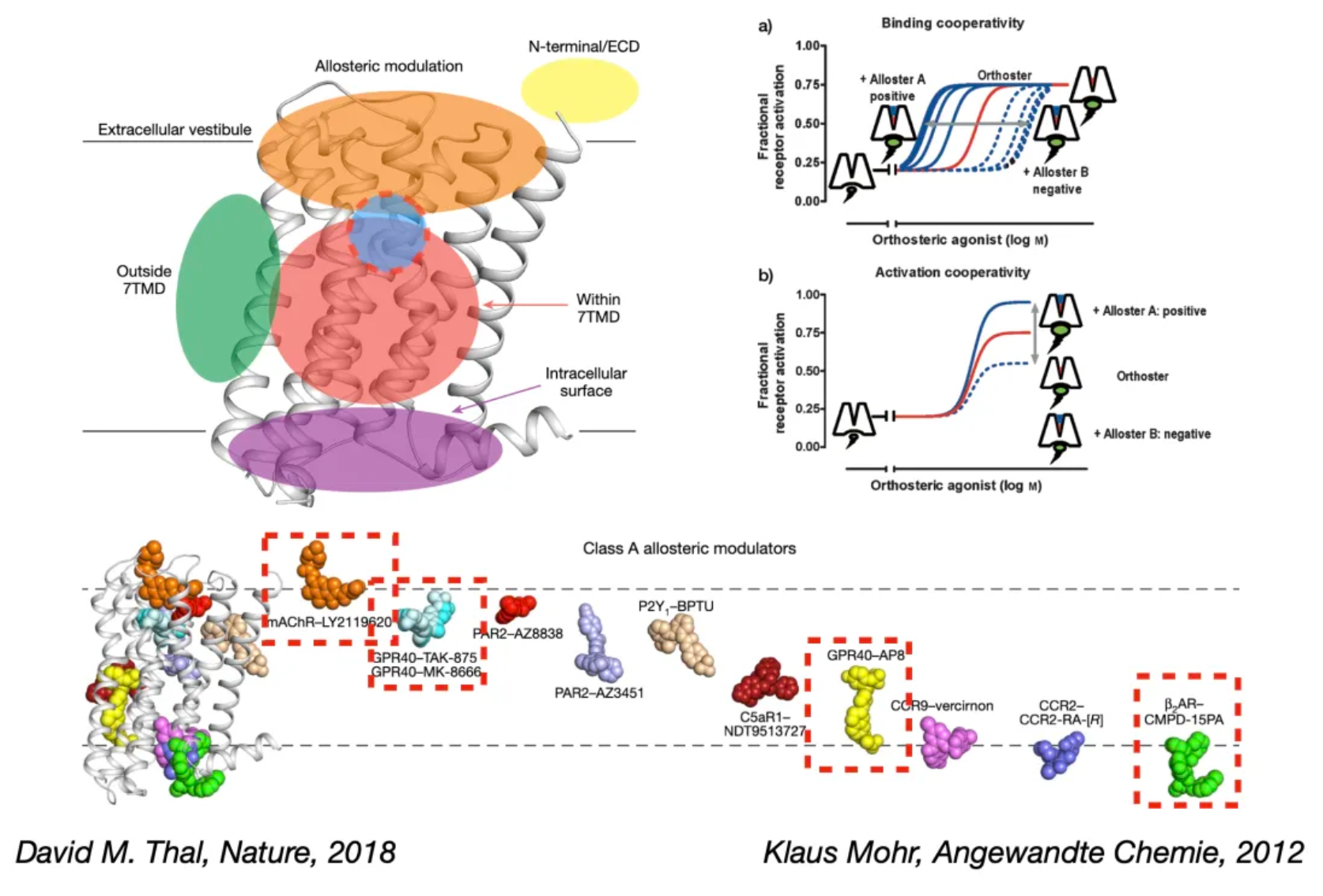
Allosteric ligands can regulate the activity of orthosteric ligands by selecting different conformations of the GPCR while allowing the orthosteric molecule to co-bind. Therefore, they are referred to as "allosteric modulators." This regulatory effect is dependent on the presence of an orthosteric ligand currently bound, a phenomenon also known as "probe dependency." Furthermore, the magnitude of this effect is limited by the cooperativity between two binding sites on the co-bound receptor.
Bitopic ligands
Bitopic ligands of GPCRs (G protein-coupled receptors) refer to a single chemical entity composed of two pharmacophoric groups covalently linked together. If these two pharmacophoric groups are identical, the ligand is defined as a homobivalent bitopic ligand, while linking two different pharmacophores results in a heterobivalent bitopic ligand. Both homobivalent and heterobivalent bitopic ligands are being developed for achieving higher affinity, receptor subtype selectivity, and biased activation of downstream signaling pathways. They are also increasingly used as a means to study receptor dimerization.
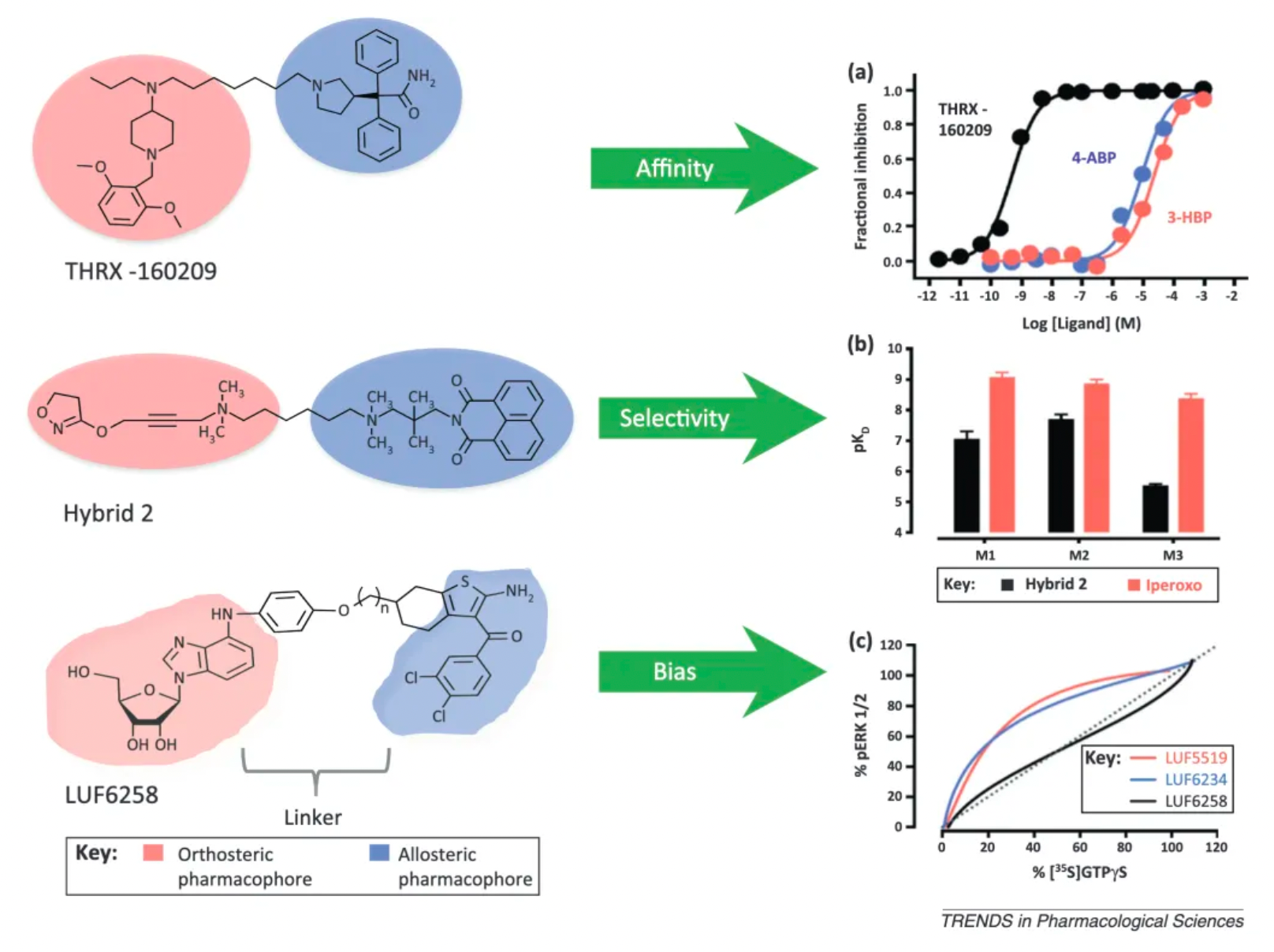
For example, THRX-160209 not only exhibits enhanced affinity but also demonstrates greater selectivity for the M2 mAChR subtype over other subtypes, which is superior to its individual ligand components. This suggests that bitopic design can contribute to specifically targeting a particular receptor subtype, reducing nonspecific effects on other subtypes.
Likewise, the bitopic ligand LUF6258, formed by linking an adenosine-derived orthosteric agonist LUF5519 with a positive allosteric modulator, exhibits a distinctive signaling bias profile in ERK1/2 phosphorylation and [35S] GTPγS binding assays compared to LUF5519 or the similar compound LUF6234 with a shorter linker.
Salmeterol is a long-acting β2 adrenergic receptor agonist that is widely used clinically as a prescription medication for the relief of symptoms in asthma and chronic obstructive pulmonary disease (COPD). In comparison to the short-acting β2 adrenergic receptor agonist salbutamol, Salmeterol exhibits a longer duration of action due to its unique bifunctional structure. Besides containing a pharmacophore that mimics the endogenous β2 adrenergic receptor ligand epinephrine, Salmeterol also features an alkyloxyphenyl tail composed of a phenolic ring attached to an 11-atom ether chain. The pharmacophore is responsible for receptor activation by binding to the orthosteric site, while the alkyloxyphenyl tail is believed to interact with an additional (allosteric) site, facilitating further interactions that are pivotal for the subtype selectivity and slow dissociation rate of the receptor.
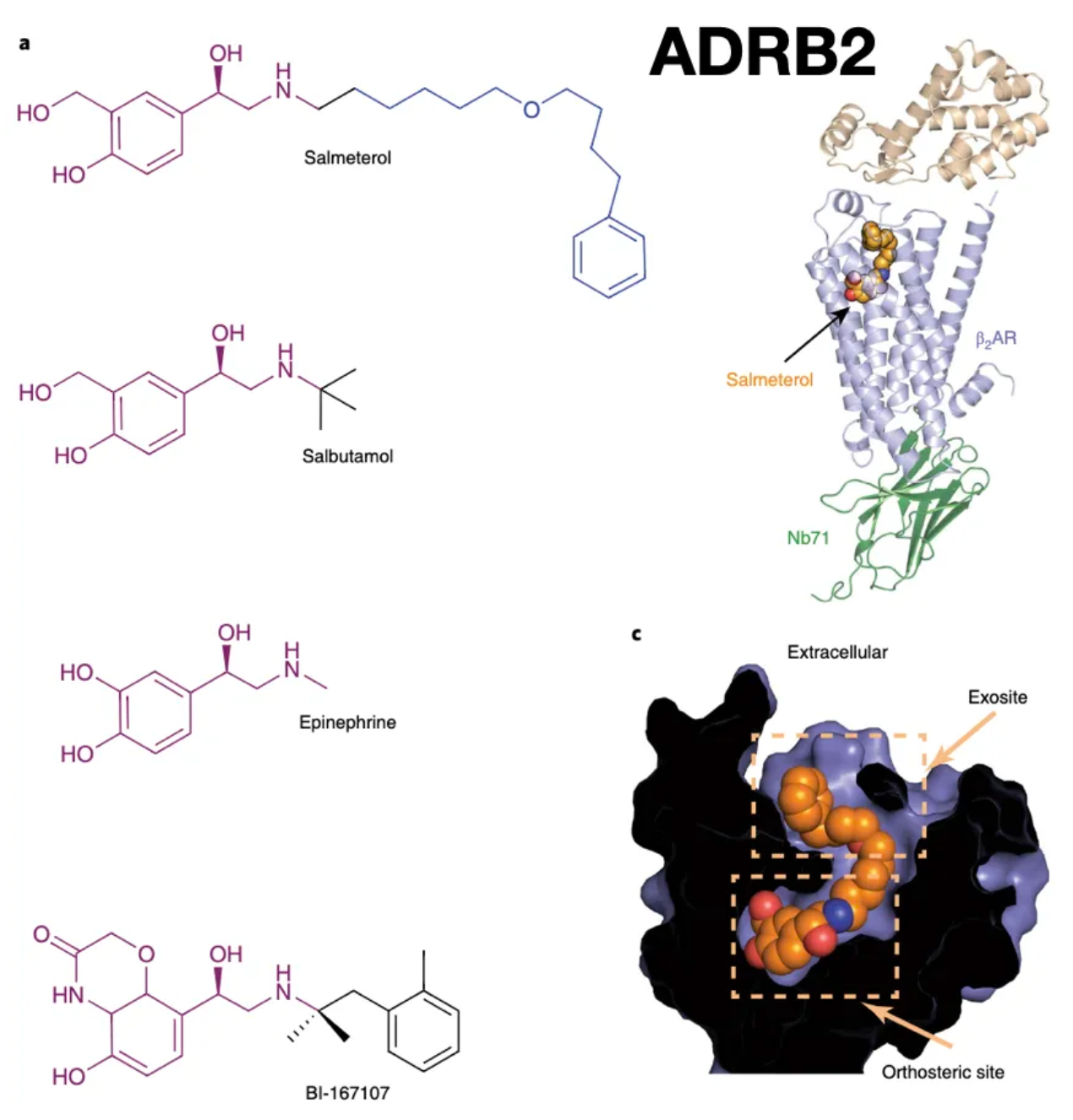
Such ligands offer new possibilities for the precise modulation of GPCR-mediated physiological processes and aid in the development of safer and more effective drugs. However, designing bifunctional ligands also poses multiple challenges, including how to precisely select and combine the orthosteric and allosteric pharmacophores, as well as predicting and optimizing their behavior within the complex receptor dynamics environment.