Boehringer Ingelheim reports encouraging outcomes from the initial global study on diabetic macular ischemia
Boehringer Ingelheim has revealed encouraging results from its HORNBILL Phase I/IIa trial for BI 764524. This study marks the first of its kind, investigating a prospective therapy for individuals suffering from diabetic macular ischemia. Results indicate that BI 764524, when administered intravitreally in both single and multiple doses, demonstrated good tolerability. The treatment successfully met its primary safety objectives and exhibited preliminary indications of possible effectiveness.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Diabetic Macular Ischemia (DMI) is a frequent, permanent result of diabetic retinopathy that often results in vision loss. It occurs when the central retina's light-sensitive tissues lack sufficient blood supply for an extended period. Currently, there are no approved therapies specifically for DMI.
The typical treatment approach for severe diabetic retinopathy (DR) involves intravitreal injections of anti-VEGF agents or more aggressive laser procedures. Despite these interventions, some patients continue to experience disease progression. The experimental drug BI 764524 targets the Sema3A pathway, offering a new therapeutic approach to revascularize affected regions and possibly surpassing the constraints of traditional treatments like anti-VEGF therapies and laser procedures.
“The impact of vision loss from conditions including diabetic retinopathy and DMI profoundly affects individuals’ quality of life. These findings represent a significant advancement towards our goal of creating precise treatments that are timely and tailored to each patient to avert vision loss before the damage becomes permanent," commented Ulrike Graefe-Mody, Ph.D., the leader of Retinal Health at Boehringer Ingelheim. “The initiation of a Phase IIb trial to test BI 764524’s safety and effectiveness is highly anticipated.”
The HORNBILL submission is among 23 abstracts presented at the Association for Research in Vision and Ophthalmology Annual Meeting 2024. Boehringer Ingelheim's presentations will cover a broad spectrum of research in retinal health, including studies on retinal non-perfusion, diabetic retinopathy, geographic atrophy, and neovascular age-related macular degeneration.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
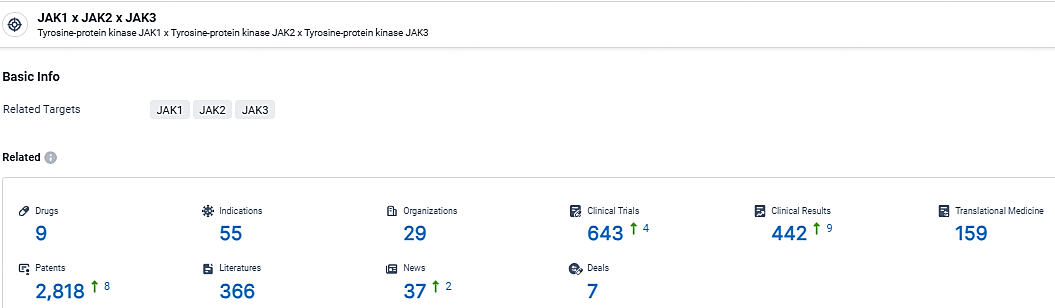
According to the data provided by the Synapse Database, As of May 7, 2024, there are 9 investigational drugs for the JAK1 and JAK2, and JAK3 targets, including 55 indications, 29 R&D institutions involved, with related clinical trials reaching 643, and as many as 2818 patents.
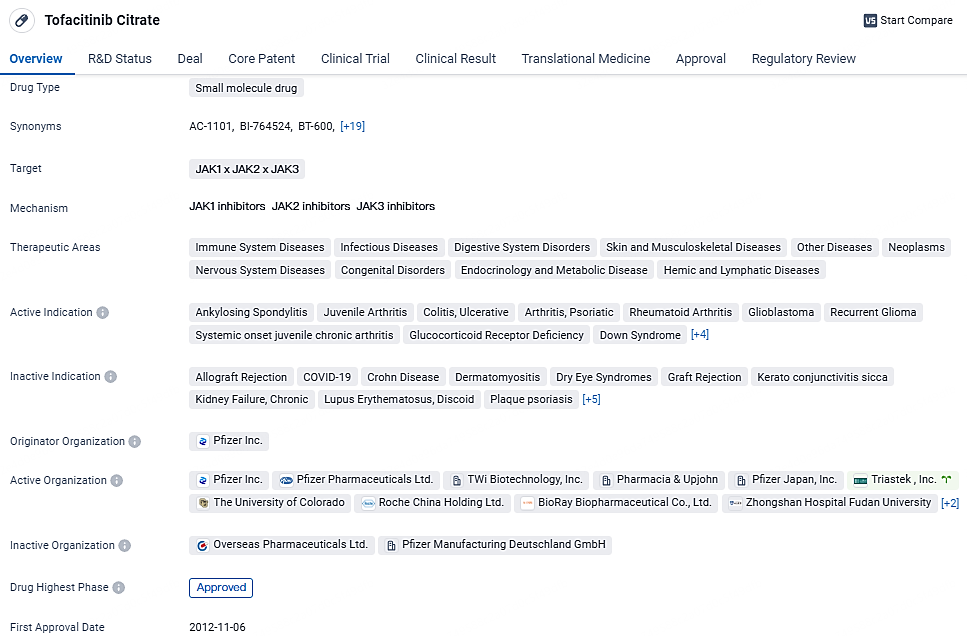
Tofacitinib Citrate is a small molecule drug developed by Pfizer Inc. that targets JAK1, JAK2, and JAK3. It has been approved for various therapeutic areas and has shown efficacy in treating multiple active indications. The drug received its first approval in the United States in 2012 and has reached the highest phase of approval globally and in China. Its regulatory status includes priority review and participation in a special review project.