C4 Therapeutics Presents Phase 1 Results on Cemsidomide as Promising IKZF1/3 Degrader
C4 Therapeutics, Inc. (C4T) (Nasdaq: CCCC), a clinical-stage biopharmaceutical firm focused on targeted protein degradation, unveiled clinical findings from the ongoing Phase 1 trial of cemsidomide, an orally available IKZF1/3 degrader, at the ASH Annual Meeting. The presentations featured a poster on cemsidomide combined with dexamethasone for multiple myeloma and an oral report on cemsidomide monotherapy for non-Hodgkin’s lymphoma. These findings underscore cemsidomide's potential as a core treatment in both multiple myeloma and non-Hodgkin’s lymphoma, where IKZF1/3 degradation is necessary.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
C4T has engineered cemsidomide to serve as a more effective and specific IKZF1/3 degrader, boasting distinctive pharmacokinetic attributes. The aim is to enhance the therapeutic index for treating multiple myeloma and non-Hodgkin’s lymphoma, either as a standalone treatment or in conjunction with other therapeutic agents in these areas.
"Cemsidomide is consistently providing clinical evidence of its potential for use in patients who are multi-refractory and as part of combination therapies across various treatment lines for a substantial number of patients with multiple myeloma or non-Hodgkin’s lymphoma," stated Len Reyno, M.D., Chief Medical Officer of C4 Therapeutics. "We are eager to use today’s data to shape clinical development strategies in both multiple myeloma and non-Hodgkin’s lymphoma, potentially unlocking the value of cemsidomide for patients requiring innovative therapies across different treatment lines."
At the ASH Annual Meeting, C4T showcased safety and anti-myeloma data indicating that cemsidomide could emerge as a top-tier IKZF1/3 degrader, serving as a preferred backbone therapy for patients with multiple myeloma where IKZF1/3 degradation is indicated. This data bolsters the future development of cemsidomide across various treatment lines in combination with other anti-myeloma therapies.
As of the data cutoff on October 11, 2024, a total of 47 patients were administered cemsidomide in combination with dexamethasone across four dosage levels (50 µg dosed MWF; 37.5 µg dosed QD; 62.5 µg QD; 75 µg QD). The patients were heavily pretreated, having received a median of six prior treatments. All patients (100 percent) had triple-class exposure, defined as exposure to at least one immunomodulatory agent, one proteasome inhibitor, and one anti-CD38 antibody. Thirty-three patients (70 percent) had prior BCMA-directed therapy. Thirty-one patients (66 percent) had prior CAR-T or T-cell engager therapy.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
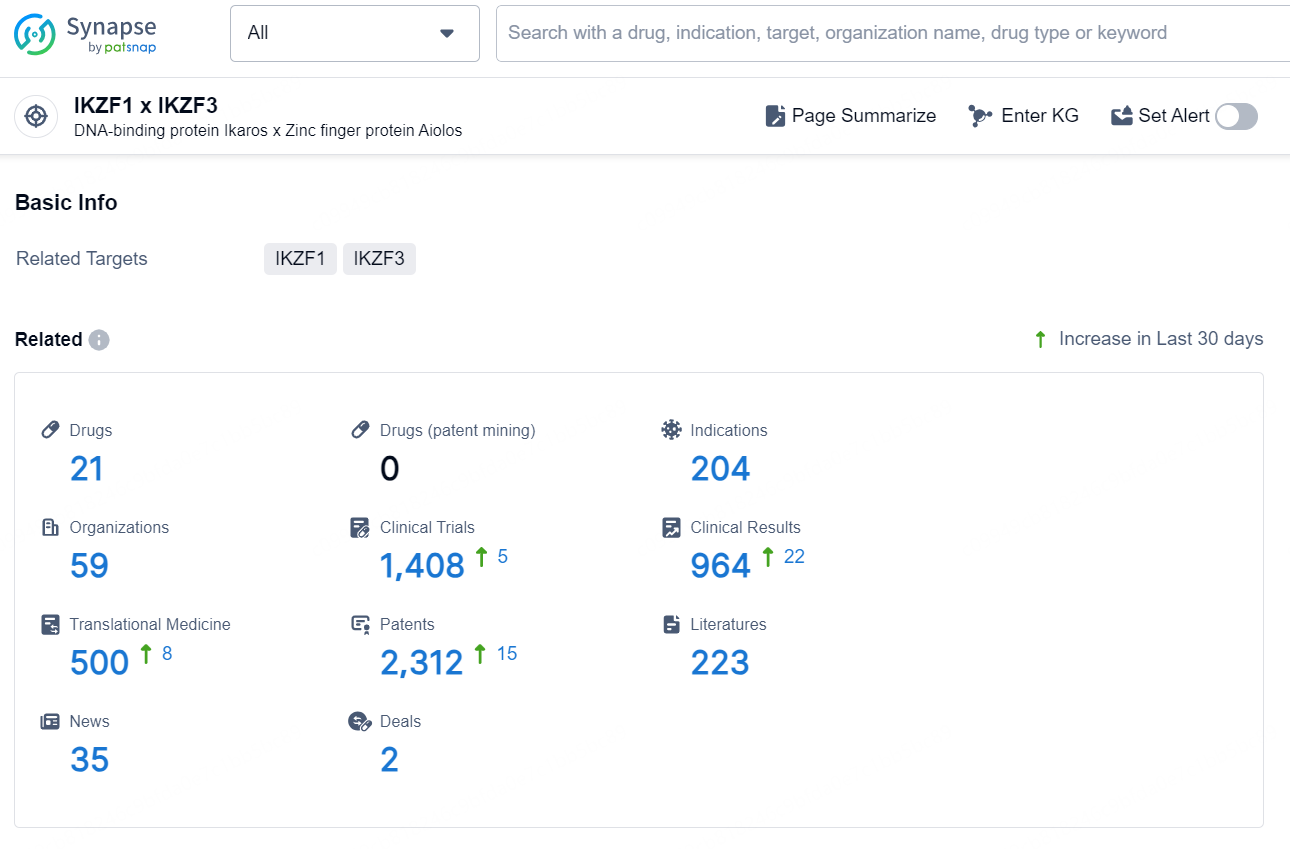
According to the data provided by the Synapse Database, As of December 12, 2024, there are 21 investigational drugs for the IKZF1 and IKZF3 target, including 204 indications, 59 R&D institutions involved, with related clinical trials reaching 1408, and as many as 2312 patents.
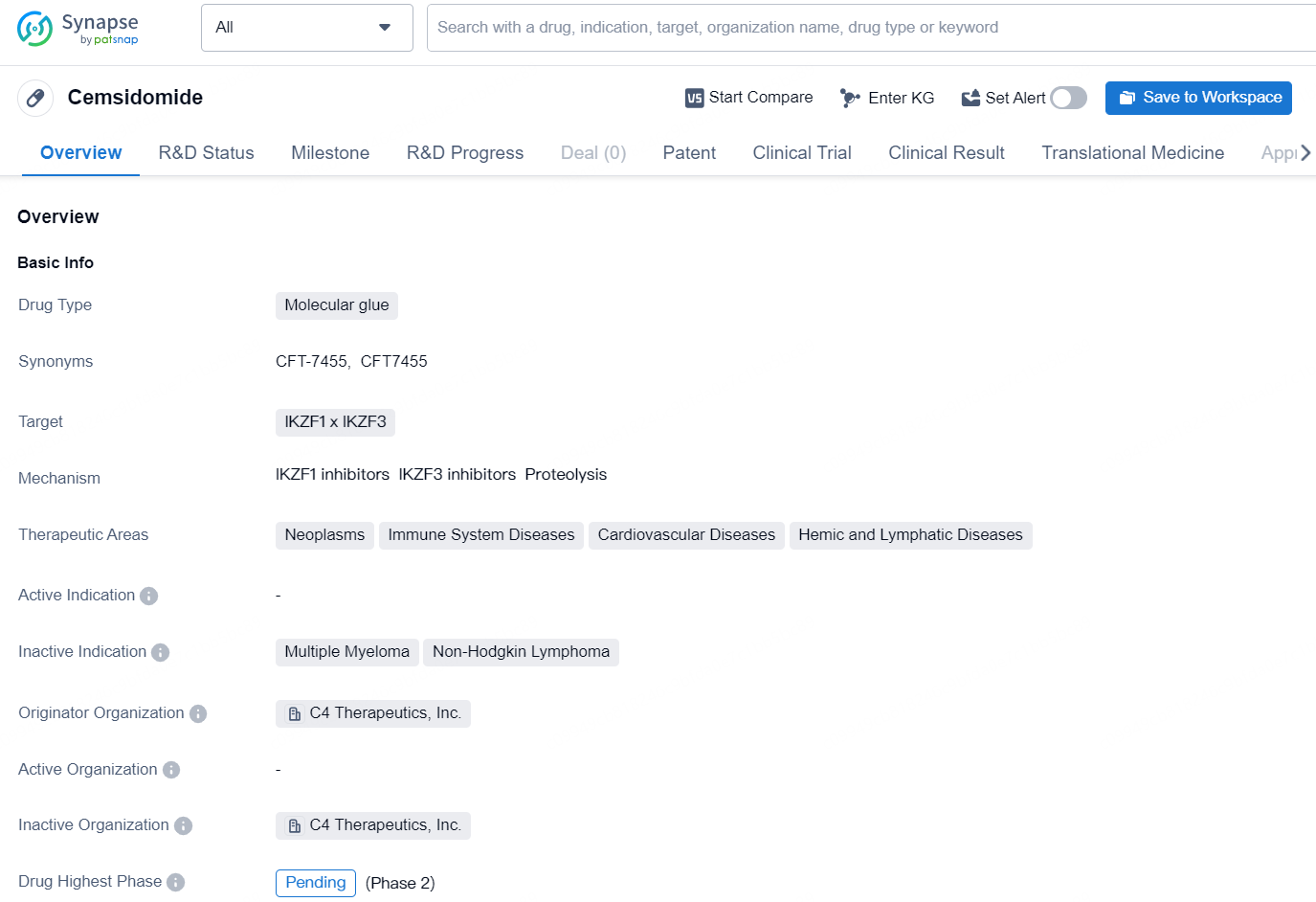
As of the information provided, Cemsidomide is classified as a molecular glue type drug that targets IKZF1 and IKZF3. It is being developed for potential therapeutic use in various areas including neoplasms, immune system diseases, cardiovascular diseases, and hemic and lymphatic diseases. The drug is currently in the pending phase for global development and is being developed by the company C4 Therapeutics, Inc.