CS5001 Clinical Findings for Advanced Lymphoma Revealed at 66th ASH Conference
CStone Pharmaceuticals, a biopharmaceutical firm driven by innovation and specializing in anti-cancer drug development, has disclosed the latest clinical findings for CS5001, an anti-ROR1 ADC and a key asset in CStone Pipeline 2.0, at the 66th American Society of Hematology (ASH) Annual Meeting. The findings underscore the drug's potential as a lymphoma treatment.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Receptor tyrosine kinase-like orphan receptor 1 (ROR1) is an embryonic tyrosine kinase-like protein involved in various pathways that enhance oncogenic signaling. ROR1 is frequently overexpressed in hematological malignancies and a wide range of solid tumors, but is minimally or not expressed in normal tissues, making it an appealing target for anti-cancer therapies. CS5001 is the first known anti-ROR1 ADC to exhibit clinical anti-tumor effects in both solid tumors and lymphomas.
Dr. Jason Yang, CEO, President of R&D, and Executive Director at CStone, stated, "We are very excited that CS5001 continues to show strong anti-tumor effects and acceptable safety and tolerability in the ongoing clinical trials. The data presented at ASH further confirm CS5001's potential, especially as a standalone treatment for patients with advanced lymphomas, many of whom have failed at least three previous treatments.
We are seeing promising anti-tumor effects in both Hodgkin's and non-Hodgkin's lymphomas, particularly with an ORR of 76.9% among the 13 assessable patients with advanced B-cell lymphoma at DL8 (125 μg/kg). As we proceed with our Phase 1b study, we will continue to assess and refine the dosage. Given CS5001's initial success in treating both aggressive and indolent lymphomas, we are confident in its broad clinical potential and strong market competitiveness. We remain dedicated to expediting the clinical development of CS5001 to bring this innovative therapy to lymphoma patients as quickly as possible."
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
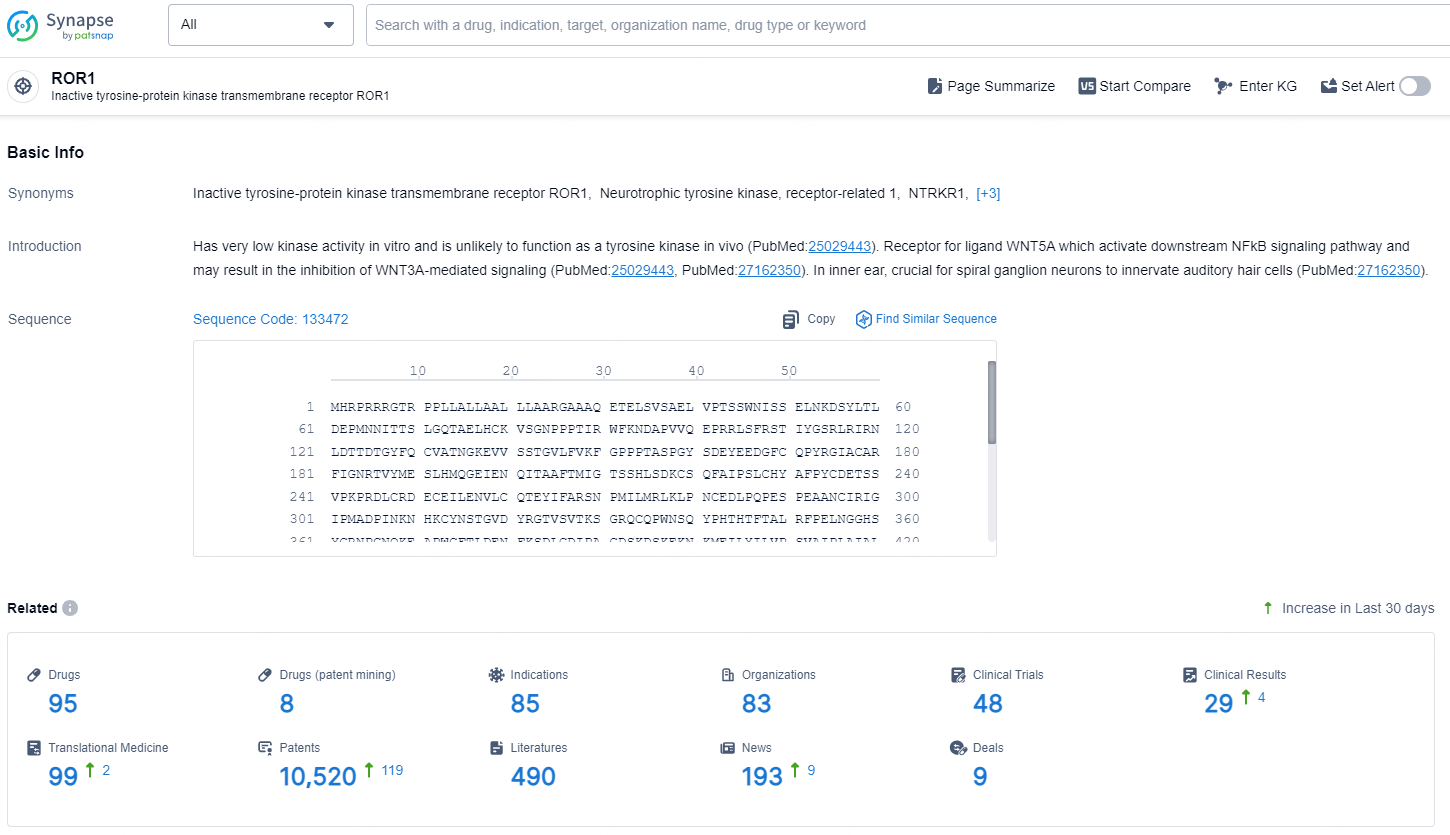
According to the data provided by the Synapse Database, As of December 12, 2024, there are 95 investigational drugs for the ROR1 target, including 85 indications, 83 R&D institutions involved, with related clinical trials reaching 48, and as many as 10520 patents.
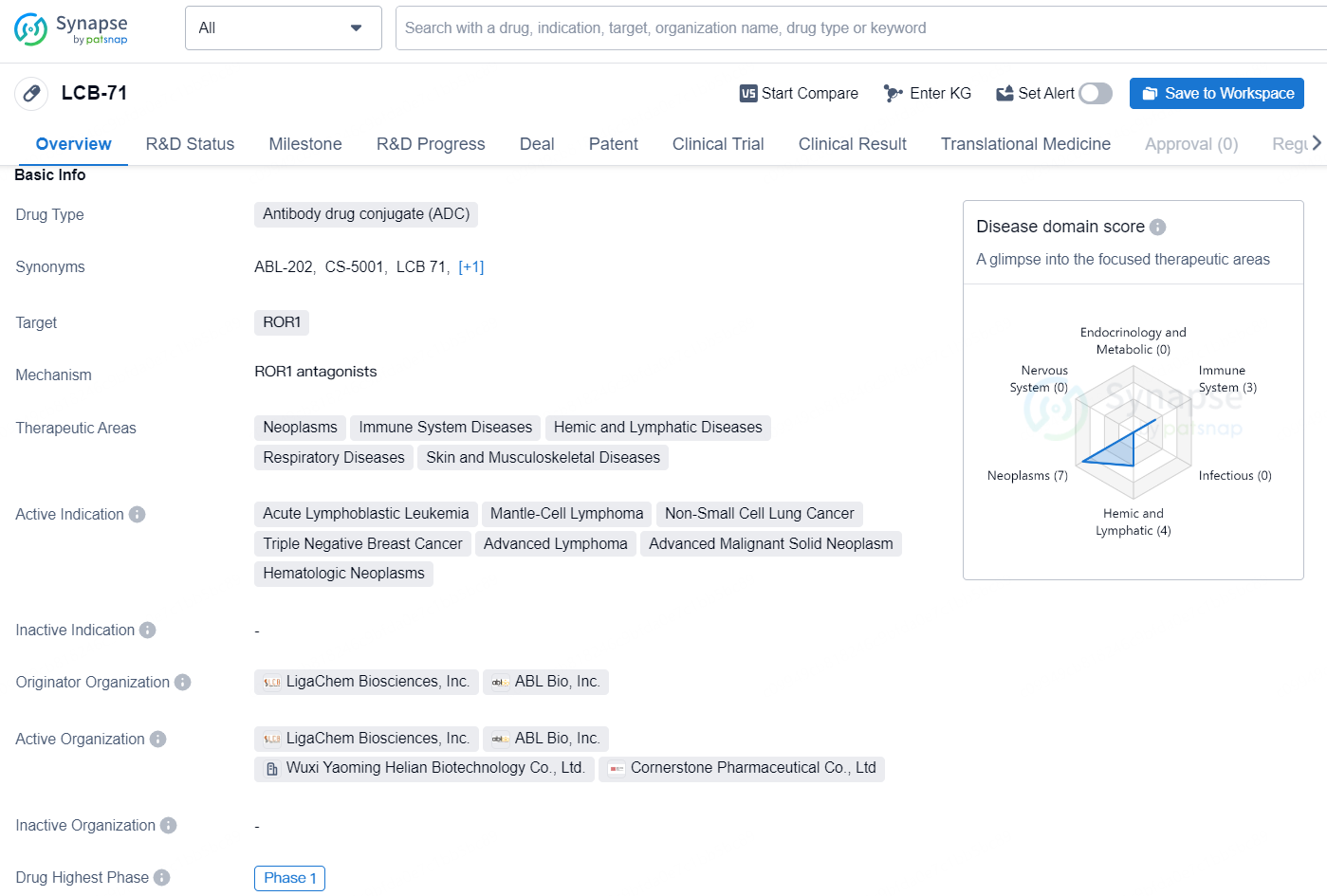
LCB-71 is an antibody drug conjugate (ADC) that targets the receptor tyrosine kinase-like orphan receptor 1 (ROR1). The therapeutic areas for this drug include neoplasms, immune system diseases, hemic and lymphatic diseases, respiratory diseases, as well as skin and musculoskeletal diseases.