Merck's Zilovertamab Vedotin and R-CHP Achieve 100% Complete Response in DLBCL Phase 2 Trial
Merck, targeting receptor tyrosine kinase-like orphan receptor 1 (ROR1). The study evaluates the use of zilovertamab vedotin alongside a regimen of cyclophosphamide, doxorubicin, and prednisone combined with rituximab (R-CHP) for patients diagnosed with untreated diffuse large B-cell lymphoma (DLBCL). In a pre-planned evaluation, the zilovertamab vedotin and R-CHP combination exhibited a remarkable complete response (CR) rate of 100% (n=15) in individuals receiving zilovertamab vedotin at a dosage of 1.75 mg/kg. This data has led to the determination of 1.75 mg/kg as the recommended dosage for Phase 3 trials. These findings are being disclosed for the first time today in an oral presentation (Abstract #578) during the 66th Annual Meeting and Exposition of the American Society of Hematology (ASH).
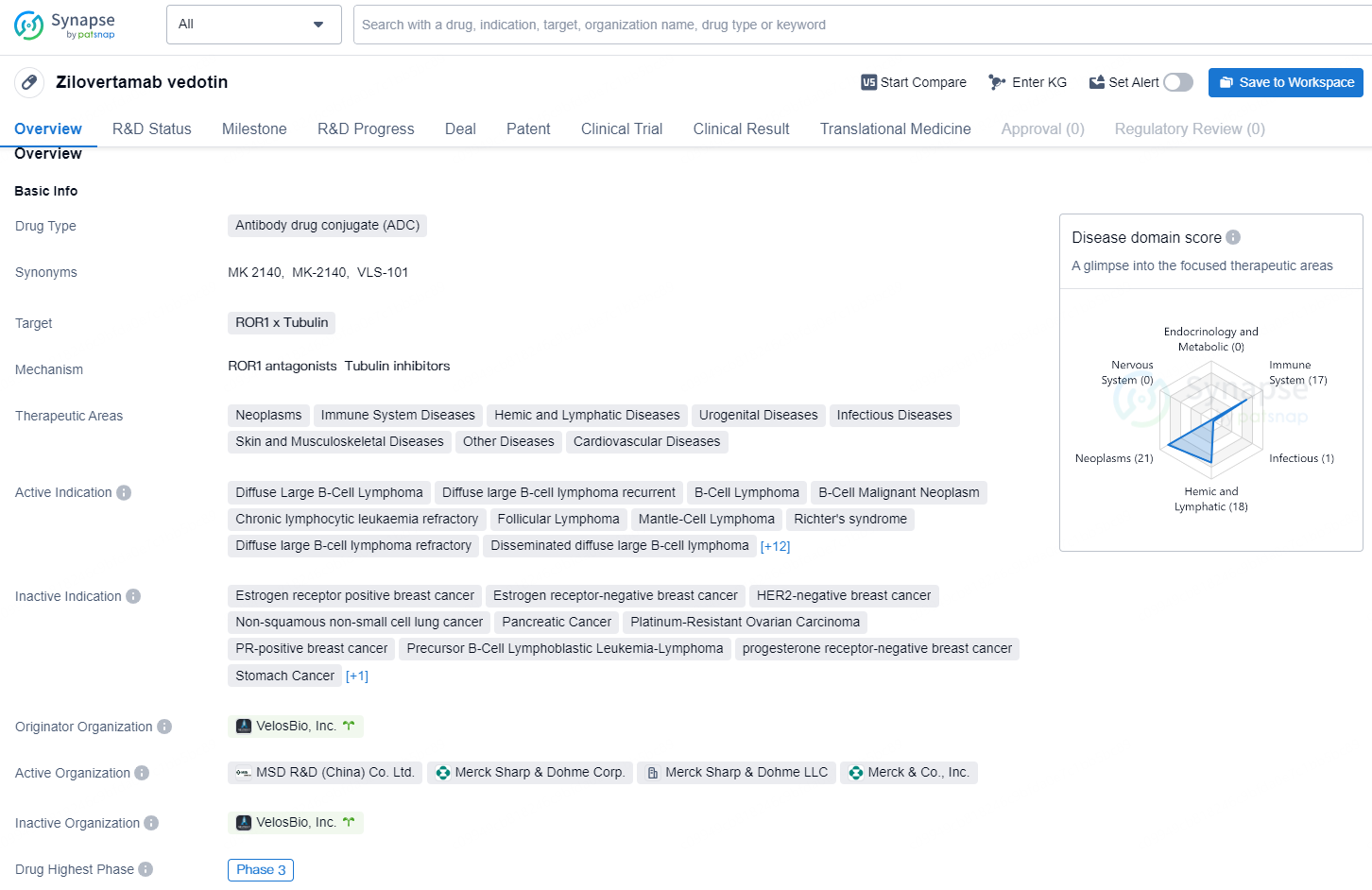
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
According to Dr. Muhit Ozcan, the principal investigator from the Ankara University School of Medicine, “There is an urgent requirement for more first-line treatment alternatives for patients diagnosed with diffuse large B-cell lymphoma, as roughly 40% still suffer from relapsed or refractory disease following the initial therapy with the current standard approach.” He emphasized that the Phase 2 waveLINE-007 trial results are encouraging and indicate the necessity for further investigation in larger patient populations to meet this critical unmet demand.
Dr. Gregory Lubiniecki, vice president of oncology clinical research at Merck Research Laboratories, commented, “We are encouraged by the early positive findings from the Phase 2 waveLINE-007 trial, where zilovertamab vedotin showed a very promising response rate alongside an acceptable safety profile when used with the standard treatment.” He expressed excitement about progressing the research of this investigational antibody-drug conjugate targeting ROR1, believing it holds significant promise in various hematologic cancers.
At the 66th ASH Annual Meeting and Exposition, Merck is presenting data from over 20 abstracts covering a wide array of hematologic malignancies, showcasing a rich portfolio of investigational assets incorporating innovative modalities from their hematology pipeline.
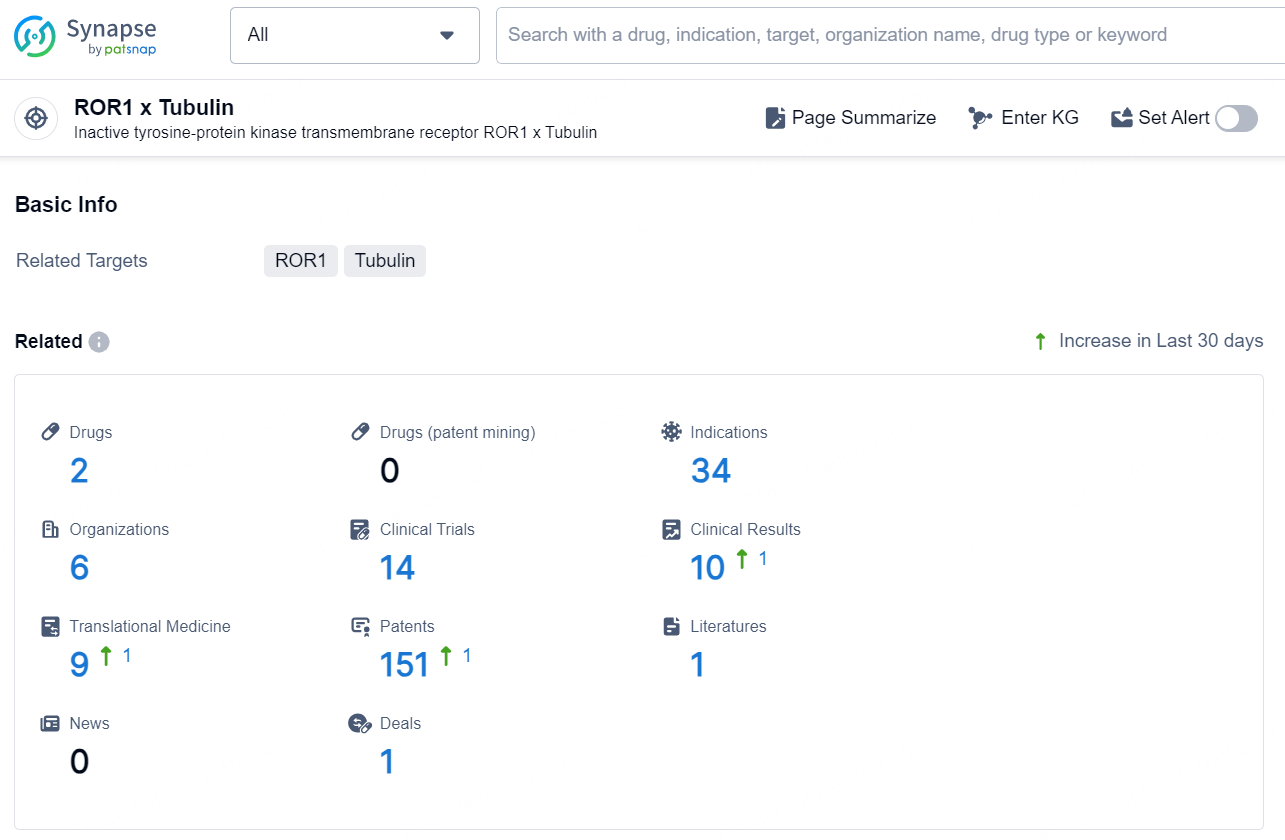
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of December 11, 2024, there are 2 investigational drugs for the ROR1 x Tubulin target, including 34 indications, 6 R&D institutions involved, with related clinical trials reaching 14, and as many as 151 patents.
Zilovertamab vedotin is an antibody drug conjugate (ADC) that targets ROR1 and Tubulin. This drug is being developed for the treatment of various therapeutic areas such as Neoplasms, Immune System Diseases, Hemic and Lymphatic Diseases, Urogenital Diseases, Infectious Diseases, Skin and Musculoskeletal Diseases, Other Diseases, and Cardiovascular Diseases.