Innovent Reveals Phase 1 Trial Results for Anti-CLDN18.2 ADC in Pancreatic Cancer at ESMO Asia 2024
Innovent Biologics, Inc. (HKEX: 01801), a leading biopharmaceutical firm that specializes in the development, manufacturing, and marketing of high-quality therapeutic agents targeting oncology, cardiovascular and metabolic diseases, autoimmune disorders, ophthalmology, and other significant health conditions, has presented updated findings from a Phase 1 clinical trial of IBI343, a novel anti-CLDN18.2 antibody-drug conjugate, for advanced pancreatic ductal adenocarcinoma (PDAC) at the ESMO Asia Congress 2024. The data underscored the remarkable efficacy and favorable safety profile of IBI343 in a larger group of CLDN18.2-positive patients who had received prior treatment for PDAC.
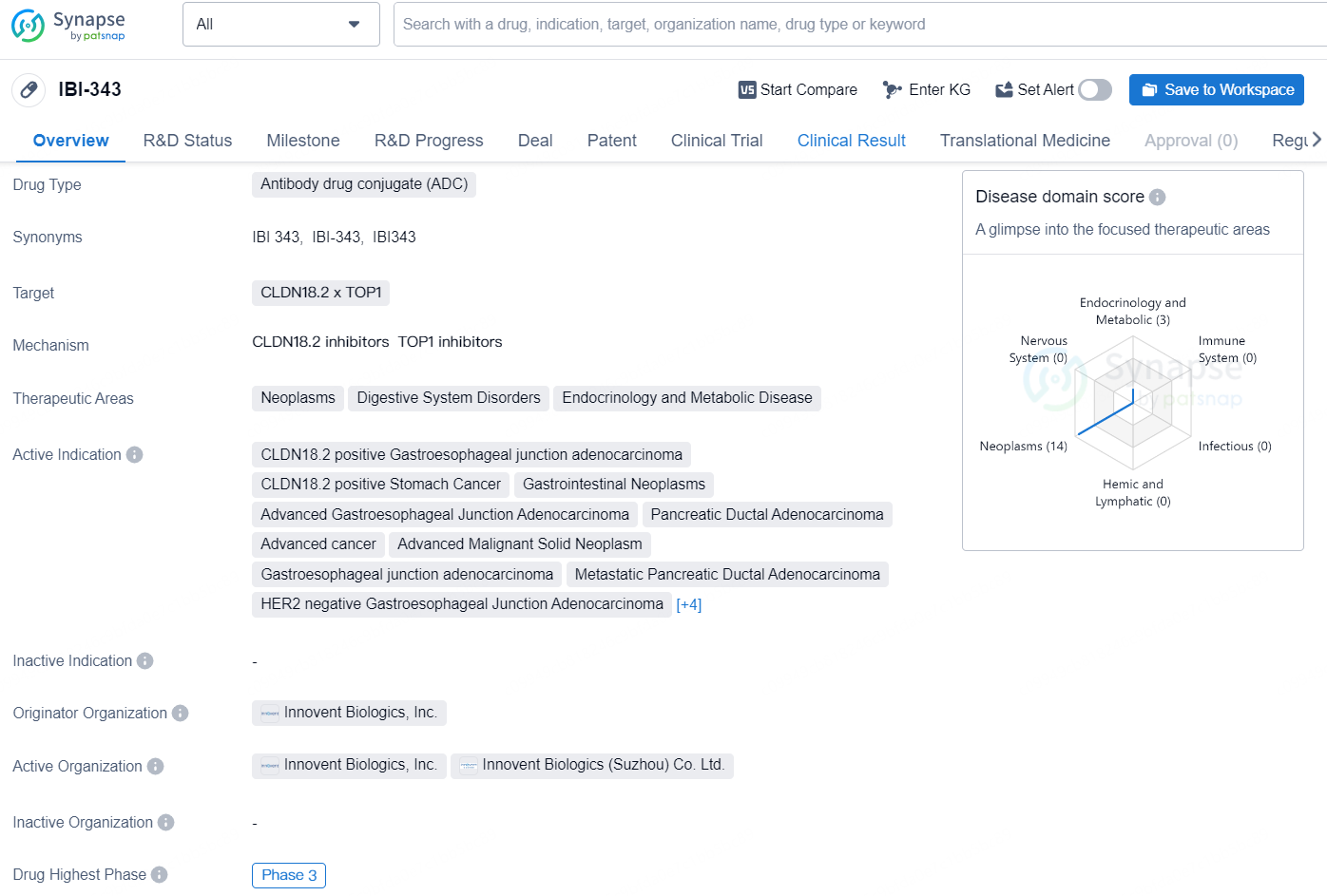
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Pancreatic cancer ranks among the most aggressive cancers globally. Most individuals receive their diagnosis at advanced stages, often developing resistance to conventional chemotherapy, which contributes to a 5-year survival rate below 10%. GLOBOCAN 2022 reports around 510,000 new pancreatic cancer cases annually, resulting in approximately 467,000 deaths worldwide, with China reporting 120,000 new cases and 110,000 deaths each year.
This Phase 1/1b trial (NCT05458219) is a multi-regional, dose-escalation and expansion clinical study. Initial findings were shared at the 2024 ASCO meeting, while the updated outcomes from the dose-expansion cohort were unveiled during the 2024 ESMO Asia Congress.
As of September 6, 2024, 43 patients with CLDN18.2-positive advanced pancreatic ductal adenocarcinoma (PDAC), defined as having at least 60% of tumor cells showing membranous staining of intensity ≥1+ by immunohistochemistry (IHC), were treated with IBI343 at a dose of 6 mg/kg every three weeks as a monotherapy. All participants had undergone at least one line of prior therapy, with 60.5% having received two or more lines of treatment for cancer.
Out of these, 43 patients were included in the efficacy evaluation, yielding an overall objective response rate (ORR) of 32.6%, a confirmed objective response rate (cORR) of 23.3%, and a confirmed disease control rate (cDCR) of 81.4%.
At the data cutoff, among the 10 patients who achieved cORR, 4 had experienced disease progression. The median duration of response (mDoR) was recorded at 7.0 months (range 4.0 - not calculated), with a 6-month duration of response rate of 63%. Progression-free survival (PFS) events occurred in 26 patients, leading to a median progression-free survival (mPFS) of 5.3 months (range 4.1-7.4), while overall survival (OS) data remain immature.
The updated safety results indicated that IBI343 demonstrates a favorable safety profile, with consistently low gastrointestinal toxicity and no new safety concerns emerging. Treatment-emergent adverse events (TEAEs) were reported by 97.7% of participants, with the most frequently observed TEAEs being anemia, reduced neutrophil count, decreased appetite, nausea, and reduced white blood cell count. A total of 51.2% of participants experienced TEAEs rated ≥ grade 3, with no instances of ≥ grade 3 nausea or vomiting reported. Additionally, none of the TEAEs resulted in fatalities.
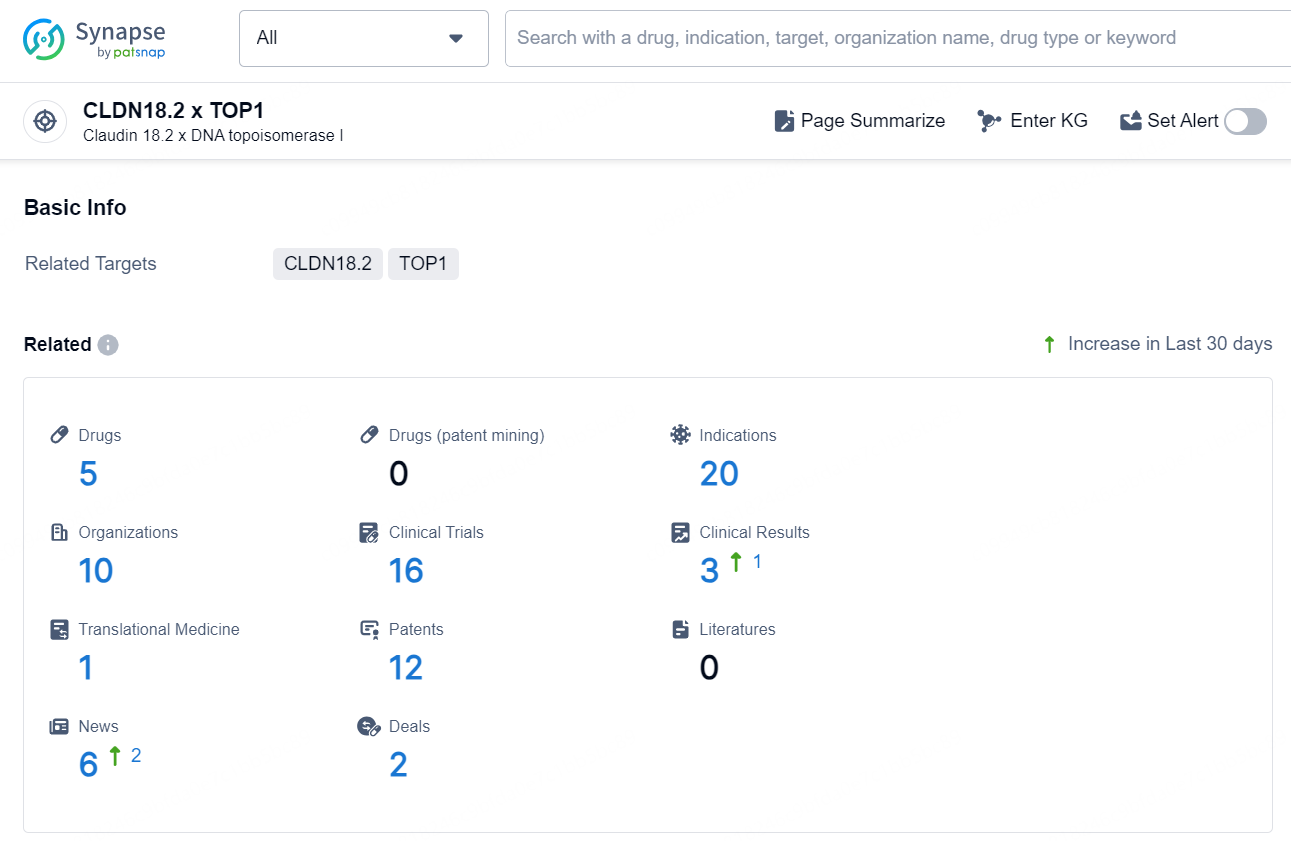
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Chemical, As of December 11, 2024, there are 5 investigational drugs for the CLDN18.2 x TOP1 target, including 20 indications, 10 R&D institutions involved, with related clinical trials reaching 16, and as many as 12 patents.
The drug IBI-343 is an antibody drug conjugate (ADC) that targets CLDN18.2 and TOP1. It is intended for use in the treatment of neoplasms, digestive system disorders, and endocrinology and metabolic diseases.