Mirikizumab: First IL23p19 Antagonist with Long-Term Data in Ulcerative Colitis and Crohn’s Disease
Eli Lilly and Company revealed findings from two extensive, multi-year Phase 3 trials indicating that patients receiving mirikizumab maintained consistent, prolonged remission in two forms of inflammatory bowel diseases: ulcerative colitis and Crohn’s disease.
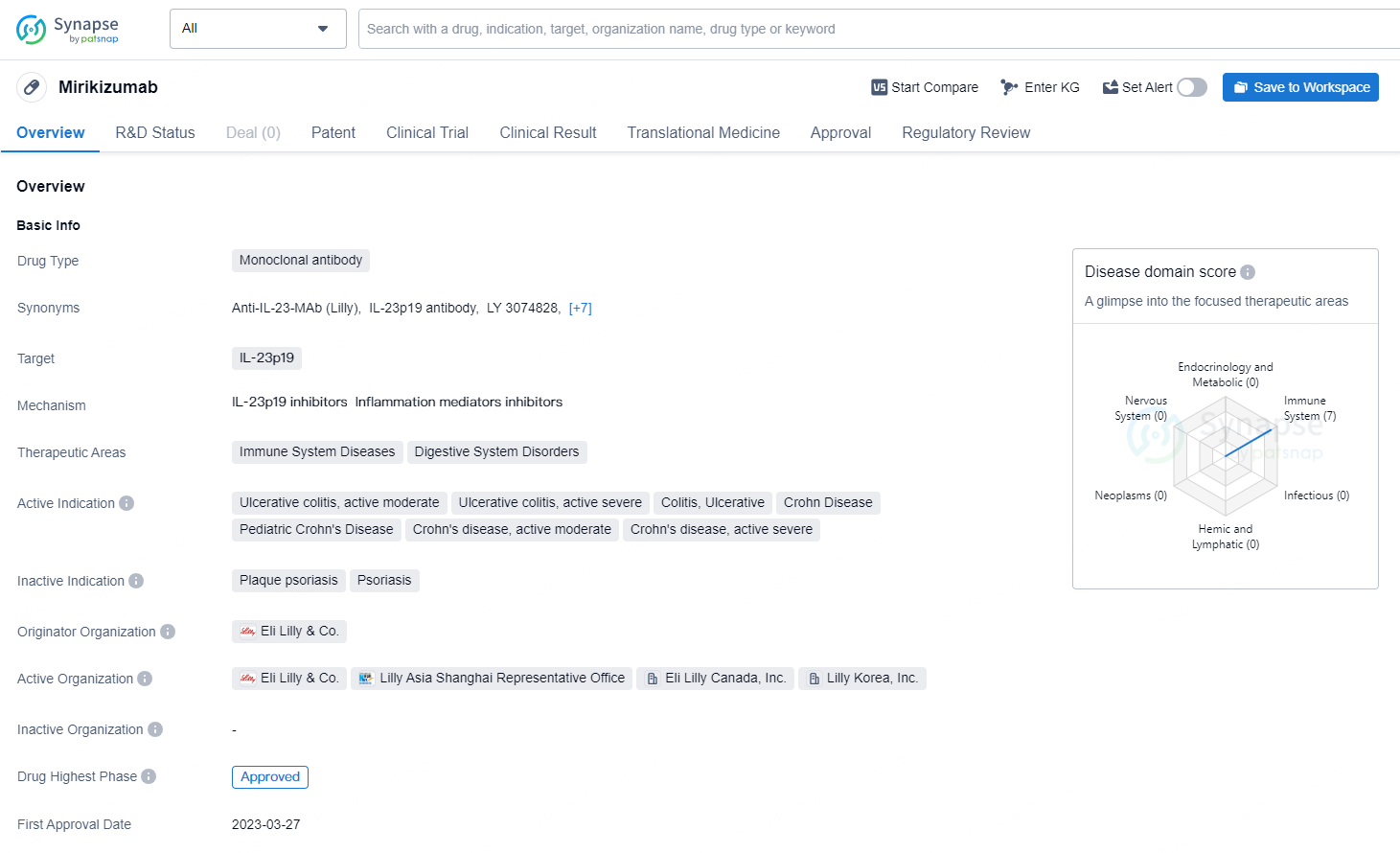
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Data from two clinical studies, LUCENT-3 focused on moderately to severely active ulcerative colitis (UC) and VIVID-2 concerning moderately to severely active Crohn’s disease, will be presented at the American College of Gastroenterology Annual Meeting scheduled for October 25-30, 2024, in Philadelphia.
Mirikizumab functions as an interleukin-23p19 (IL23p19) antagonist, specifically targeting the p19 subunit of IL-23, thereby preventing its engagement with the IL-23 receptor. The excessive activation of the IL-23 pathway significantly contributes to the inflammatory processes underlying UC and Crohn's disease. The resulting inflammation can provoke severe symptoms, such as bowel urgency, which may detrimentally affect patients' health-related quality of life, along with posing risks for irreversible health issues if not managed appropriately.
In the U.S., Mirikizumab has been authorized for treating adults with moderately to severely active UC and is currently being evaluated by the U.S. Food and Drug Administration (FDA) for use in moderately to severely active Crohn’s disease.
“Mirikizumab stands out as the first IL23p19 antagonist to deliver long-term efficacy data spanning multiple years for both ulcerative colitis and Crohn’s disease,” remarked Mark Genovese, M.D., senior vice president at Lilly Immunology development. “This milestone underscores our dedication to supporting individuals with immune-related disorders achieve long-lasting remission and alleviate their disease-related burdens.”
“Even with the progress made, individuals dealing with ulcerative colitis and Crohn’s disease continue to search for therapies that effectively manage challenging symptoms like bowel urgency and deliver sustained outcomes,” stated Bruce Sands, M.D., M.S., Dr. Burrill B. Crohn Professor of Medicine and Chief of the Dr. Henry D. Janowitz Division of Gastroenterology at the Icahn School of Medicine at Mount Sinai. “The multi-year results indicate that mirikizumab serves as a targeted treatment capable of promoting intestinal healing over time and enhancing key symptoms that are most significant for patients.”
Omvoh® (mirikizumab-mrkz) received FDA approval in October 2023 as the inaugural IL23p19 antagonist for treating adults with moderately to severely active UC and has also gained approval in 44 countries worldwide. Lilly is actively pursuing marketing applications for mirikizumab in the context of Crohn’s disease across various regions, including the U.S., Canada, Europe, Japan, and China, with additional global regulatory applications planned.
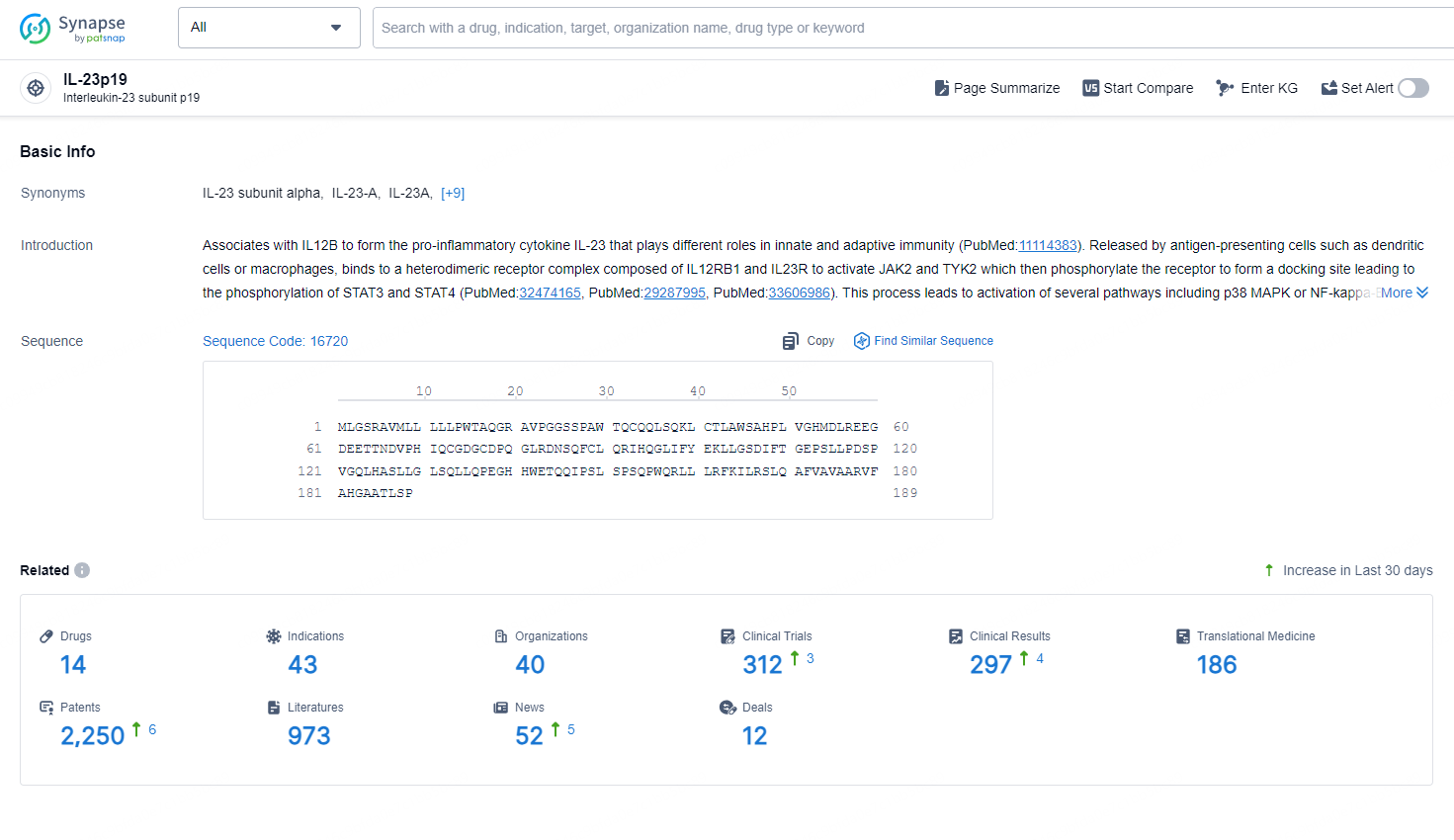
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Chemical, As of October 30, 2024, there are 14 investigational drugs for the IL-23p19 target, including 43 indications,40 R&D institutions involved, with related clinical trials reaching 312, and as many as 2250 patents.
Mirikizumab represents a significant advancement in the field of biomedicine, particularly in the treatment of various immune and digestive system disorders. Its specific targeting of IL-23p19 makes it a promising option for patients with conditions such as ulcerative colitis and Crohn's disease. With its approval and upcoming availability in Japan, the drug is positioned to have a positive impact on the management of these conditions. As a monoclonal antibody, it represents a novel approach to treatment and holds potential for further developments in the pharmaceutical industry.