Dosing of First Patient in Adicet Bio's ADI-270 Phase 1 Trial for Advanced Renal Cancer
Adicet Bio, Inc. (Nasdaq: ACET), a biotechnology firm at the clinical stage focused on the discovery and development of allogeneic gamma delta T cell therapies for cancer and autoimmune disorders, has announced that the initial patient has been administered treatment in the Phase 1 clinical trial assessing ADI-270 in individuals with metastatic or advanced ccRCC.
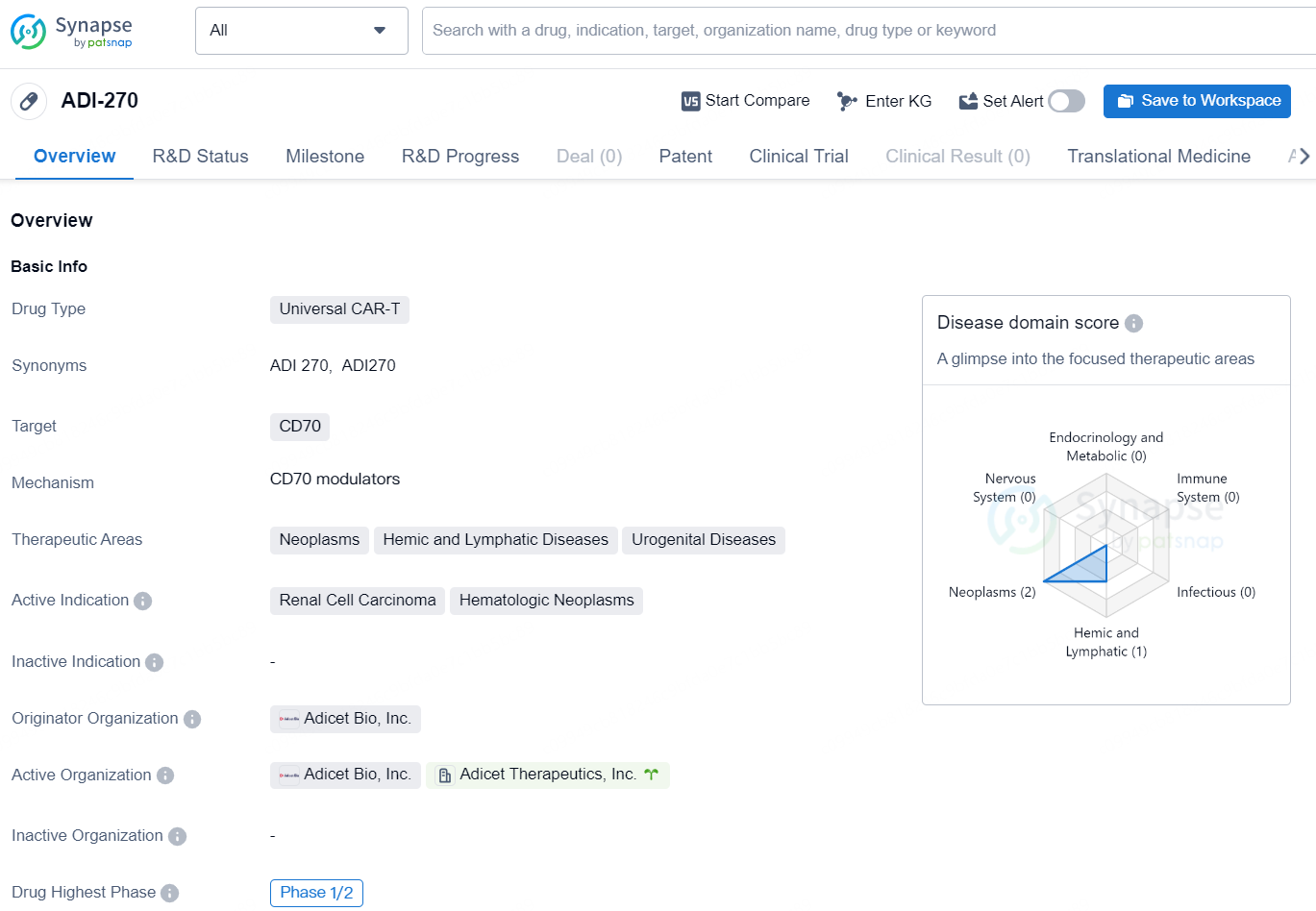
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
“Administering the first dose to a patient in our Phase 1 study of ADI-270 for advanced or metastatic ccRCC marks a pivotal achievement for Adicet as we progress with our initial gamma delta 1 CAR T cell candidate aimed at addressing a critical gap in solid tumor therapies,” stated Chen Schor, President and CEO of Adicet Bio. “There is an urgent requirement for safe and effective treatments for patients with ccRCC, the most prevalent form of kidney cancer, since existing options provide minimal advantages for those with advanced stages of the disease. Given the promising preclinical results of ADI-270 observed so far, which indicate significant tumor infiltration, resistance to the immunosuppressive tumor microenvironment, and strong activity through CAR and innate targeting, we are optimistic that ADI-270 could represent a notable step forward in treating solid tumors. We expect to share preliminary clinical findings from this study in the first half of 2025.”
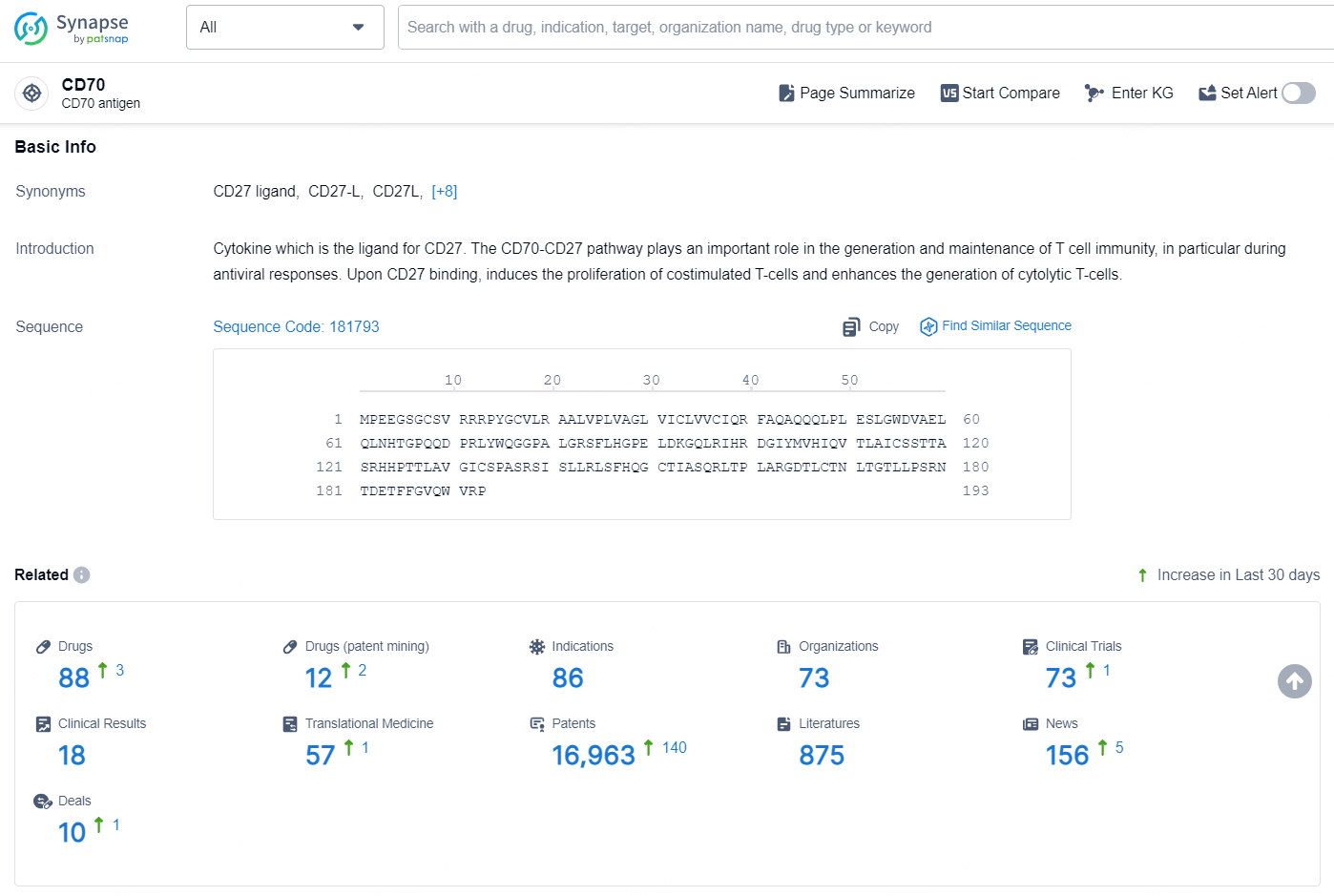
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of December 24, 2024, there are 88 investigational drugs for the CD70 target, including 86 indications, 73 R&D institutions involved, with related clinical trials reaching 73, and as many as 16963 patents.
ADI-270 is a Universal CAR-T drug developed by Adicet Bio, Inc. The drug targets CD70 and is primarily used in the treatment of Neoplasms, Hemic and Lymphatic Diseases, and Urogenital Diseases. Its active indications include Renal Cell Carcinoma and Hematologic Neoplasms. The drug is currently in Phase 1/2 of development on a global scale and has been granted Fast Track designation for regulation.