Escient Pharmaceuticals is now conducting a trial-based study on EP262, a groundbreaking oral MRGPRX2 blocker for chronic hives
Escient Pharmaceuticals, a firm engaged in the clinical-stage development of unique small molecule treatments for systemic neuroimmune conditions, recently reported the commencement of patient dosing in the CALM-CIndU trial. This open label Phase 1b clinical trial seeks to gauge the effectiveness of EP262 in individuals diagnosed with chronic inducible urticaria.
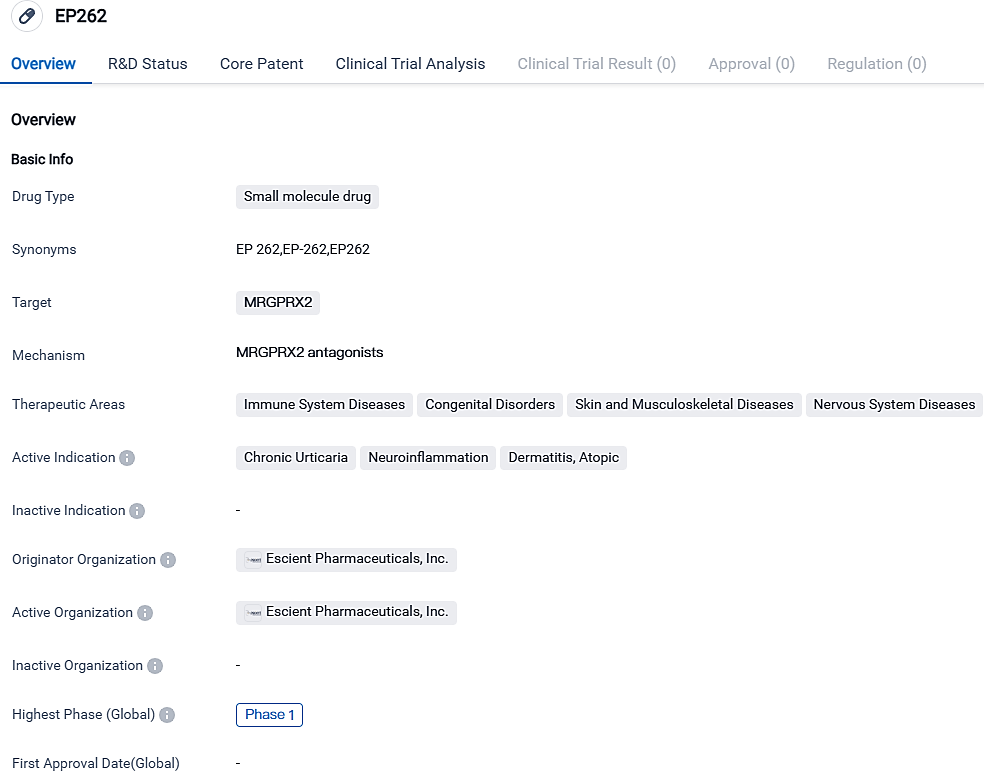
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
EP262's ability to hinder MRGPRX2 activation and mast cell degranulation presents promising prospects for treating various afflictions involving mast cell activity, primarily chronic urticaria and atopic dermatitis. This innovative and focused approach employed by EP262 opens the possibility for treating said conditions with daily oral administration while minimizing the side effects associated with conventional methods.
"Considering the fact that daily life is filled with inevitable triggers that activate mast cells, thereby making chronic inducible urticaria a crippling condition, there is an urgent need for new treatment methods," stated Dr. Martin Metz, a Dermatology and Allergy professor at Charité located in Berlin, Germany.
The CALM-CIndU clinical trial, an open-label Phase 1b study, is formulated to determine the safety, tolerability, and pharmacodynamics of EP262 in nearly 30 patients suffering from either symptomatic dermographism or cold urticaria, the two most prevalent forms of CIndU. The assessment of pharmacodynamics includes changes in disease-specific provocation thresholds from the baseline used as objective makers in diagnostic and pharmacodynamic measurement to calibrate disease severity and treatment response.
Following the successful completion of the Phase 1 human trial earlier this year, Escient's Chief Medical Officer, Christian Weyer, M.D., M.A.S, expressed, "Our team aims to embark on further clinical proof-of-concept studies in chronic spontaneous urticaria and atopic dermatitis in the impending months."
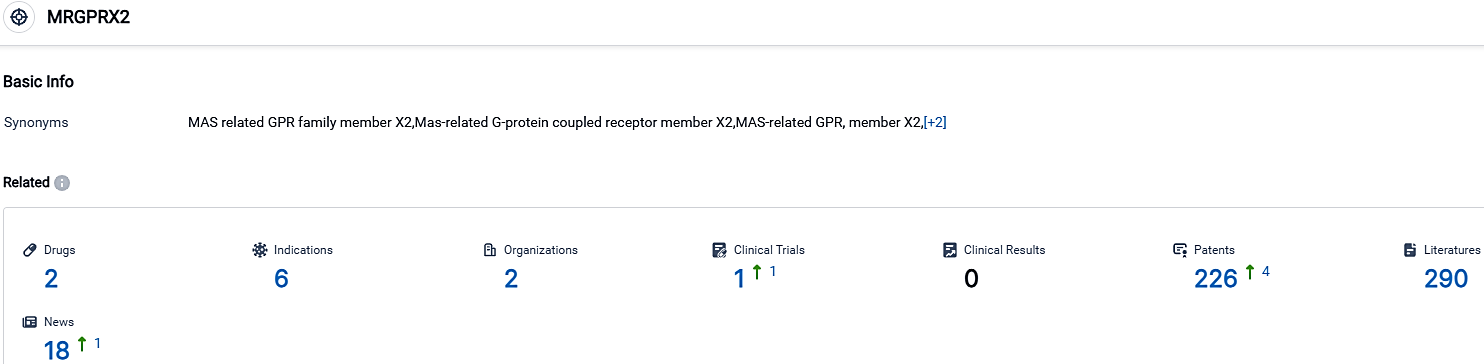
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of September 30, 2023, there are 2 investigational drugs for the MRGPRX2 target, including 6 indications,2 R&D institutions involved, with related clinical trials reaching 1,and as many as 226 patents.
EP262, a potent MRGPRX2 antagonist, is a small molecule that is extremely selective and requires only a single daily dose. This receptor is found on mast cells and can be activated by various ligands. EP262 has demonstrated promising results for the treatment of persistent urticaria, neuroinflammation, dermatitis, and other atopic diseases. More comprehensive studies and clinical trials will be required to evaluate its therapeutic effectiveness and safety.