Fasenra has received EU approval for treating eosinophilic granulomatosis with polyangiitis
AstraZeneca’s Fasenra (benralizumab) has received approval in the European Union as a supplementary therapy for adults dealing with relapsing or refractory eosinophilic granulomatosis with polyangiitis. EGPA is an uncommon, immune-mediated type of vasculitis that can lead to harm to various organs and can be lethal if not treated.
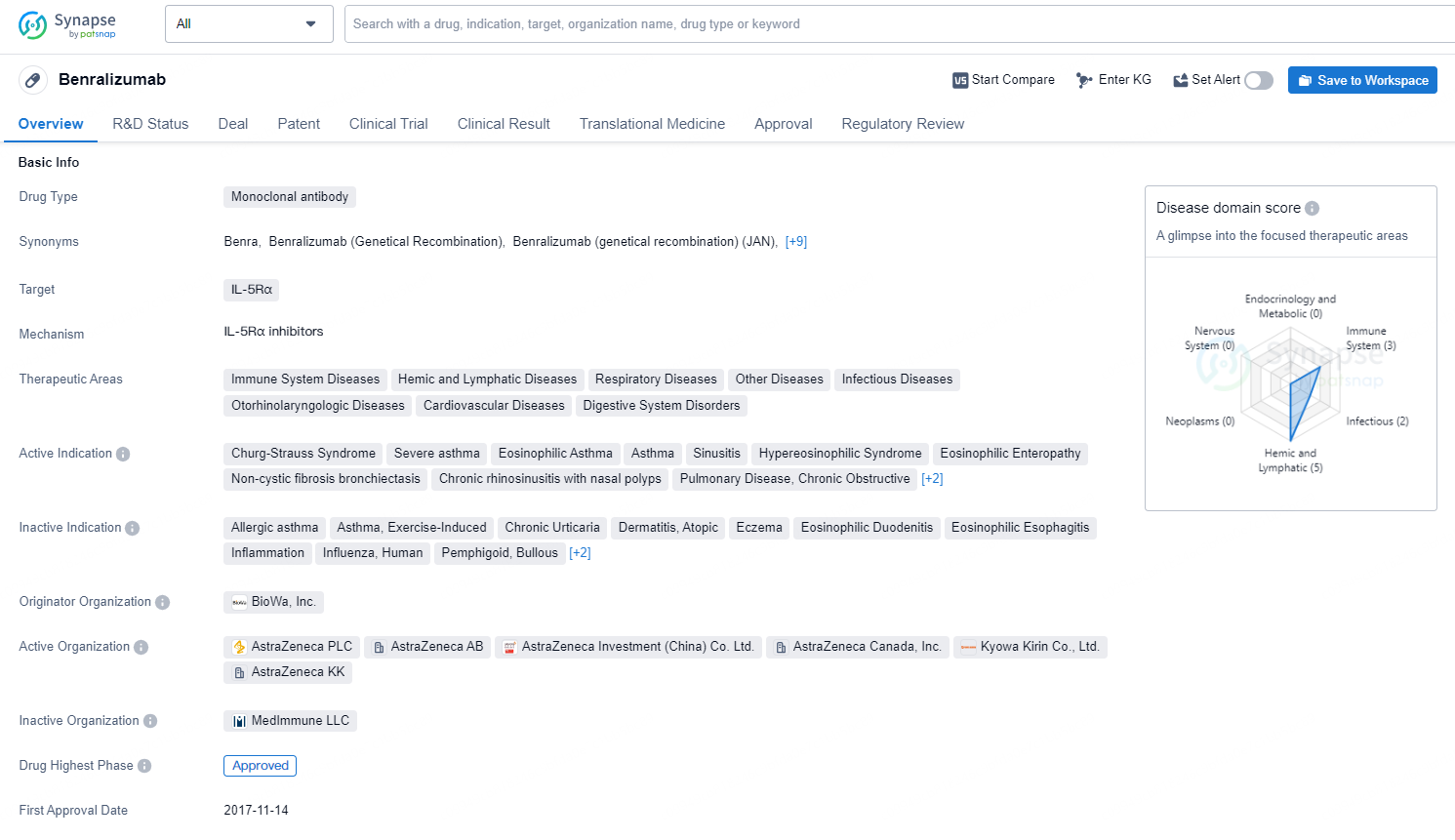
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The endorsement from the European Commission came after a favorable assessment by the Committee for Medicinal Products for Human Use and relied on encouraging findings from the MANDARA Phase III trial, which were reported in The New England Journal of Medicine. MANDARA represented the inaugural head-to-head non-inferiority study of biologic treatments in individuals with EGPA. Participants were randomized to receive either a single subcutaneous injection of 30 mg of Fasenra or three separate 100 mg subcutaneous doses of mepolizumab every month.
Bernhard Hellmich, the Principal Investigator for MANDARA, stated, "Those affected by EGPA endure severe symptoms, organ impairment, and even mortality. The approval today is a significant advancement for EGPA patients in the EU. By specifically targeting and alleviating eosinophilic inflammation using benralizumab, I aspire to see an increase in the number of patients reaching remission and a decrease in their dependence on oral corticosteroids, which can lead to serious long-term complications."
Ruud Dobber, Executive Vice President of the BioPharmaceuticals Business Unit at AstraZeneca, mentioned, "We are dedicated to supporting patients battling extremely challenging diseases. The recent approval of Fasenra, featuring its convenient once-monthly injection, represents a crucial progress for EGPA patients. Fasenra has been a well-established therapy for numerous years for thousands with severe eosinophilic asthma, and we are delighted to provide a necessary treatment option for those with EGPA in Europe."
Currently, Fasenra is approved as an additional maintenance therapy for severe eosinophilic asthma in over 80 nations, including the United States, Japan, the European Union, and China. It is also authorized for children and adolescents aged 6 and older in both the United States and Japan. In September, Fasenra received approval for EGPA in the United States.
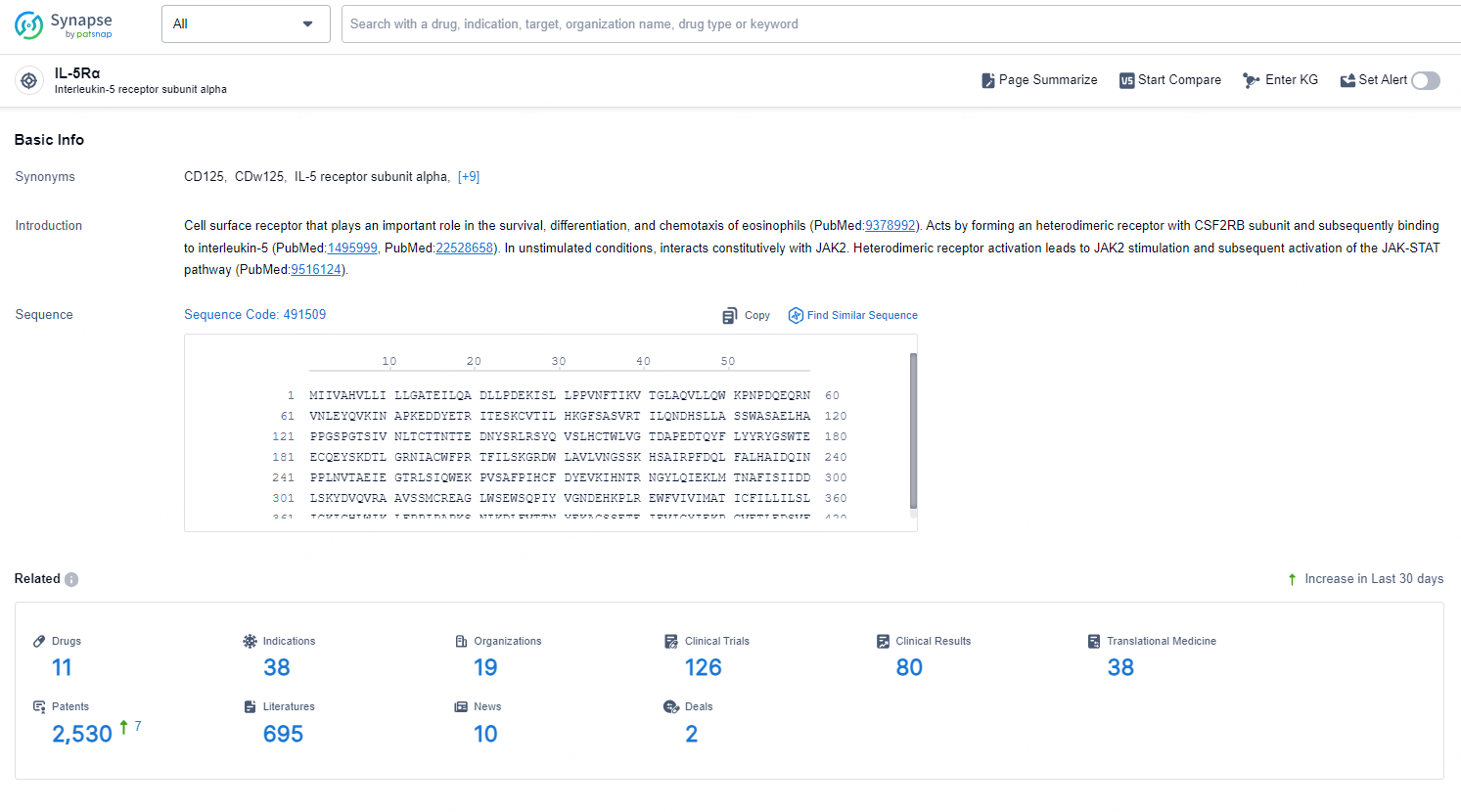
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of October 30, 2024, there are 11 investigational drugs for the IL-5Rα target, including 38 indications, 19 R&D institutions involved, with related clinical trials reaching 126, and as many as 2530 patents.
As a monoclonal antibody drug targeting IL-5Rα, Benralizumab represents a significant advancement in the treatment of asthma and other related conditions. Its approval in both the United States and China, along with its Fast Track and Orphan Drug designations, underscores the drug's potential in addressing unmet medical needs. It is expected to provide substantial benefits to patients suffering from various immune and respiratory system disorders.