FDA Approves Accent Therapeutics' IND Application for Oral DHX9 Inhibitor ATX-559
Accent Therapeutics, a biopharmaceutical firm at the forefront of developing innovative small molecule therapies for cancer, has revealed that the U.S. Food and Drug Administration (FDA) has approved its Investigational New Drug (IND) application for ATX-559, which is a pioneering DHX9 inhibitor. Furthermore, Accent has formed a Clinical Advisory Board (CAB) to assist in directing the company's clinical advancements.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
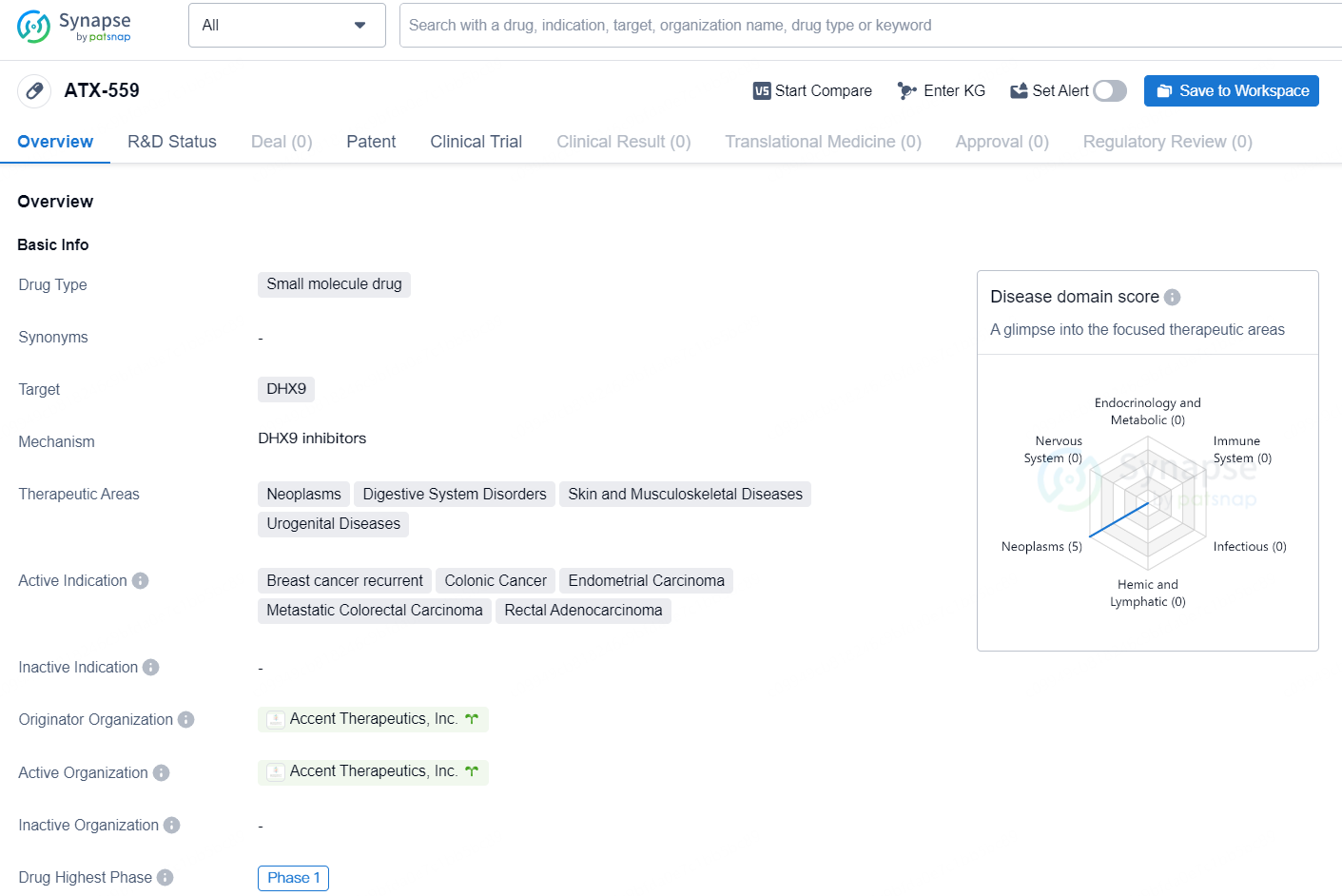
The ATX-559 Phase 1/2 study (NCT06625515) is anticipated to commence patient dosing in the last quarter of 2024, targeting individuals with BRCA1 or BRCA2 deficient breast cancer and patients presenting MSI-H and/or dMMR solid tumors, which may include specific cases of colorectal, endometrial, gastric, and other forms of cancer. This clinical trial aims to assess ATX-559 in terms of safety, tolerability, pharmacokinetics, pharmacodynamics, and initial efficacy. Further details regarding the trial can be found on the ClinicalTrials.gov website.
"The FDA's IND approval marks a significant milestone for Accent Therapeutics, allowing our lead program to enter clinical testing. This achievement not only reinforces our commitment to a scientifically driven strategy for discovering innovative therapeutic targets but also brings us closer to addressing the needs of cancer patients with substantial unmet medical requirements," stated Jason Sager, M.D., Chief Medical Officer of Accent Therapeutics. "Preclinical findings for ATX-559 have been exceptionally promising, and we look forward to investigating its potential to significantly enhance outcomes for patients battling these formidable cancers."
As Accent prepares to launch its inaugural clinical trial, the company has also formed a Clinical Advisory Board. This board comprises distinguished clinician-scientists and leaders whose valuable insights and clinical knowledge will aid the company's mission to develop transformative therapies for individuals affected by cancer.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
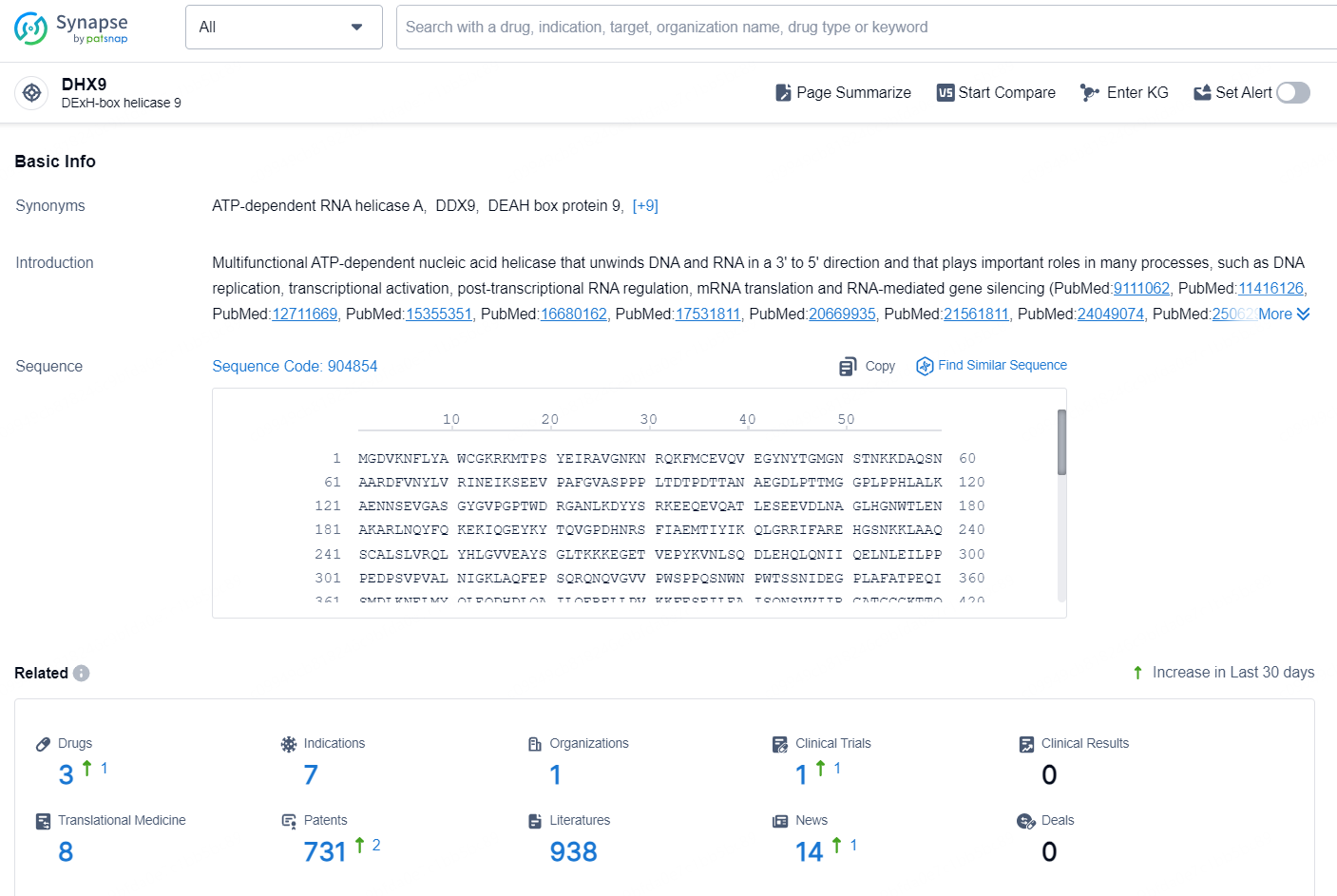
According to the data provided by the Synapse Database, As of October 29, 2024, there are 3 investigational drugs for the DHX9 target, including 7 indications, 1 R&D institution involved, with related clinical trial reaching 1, and as many as 731 patents.
ATX-559 is a small molecule drug developed by Accent Therapeutics, Inc. It targets DHX9 and is being developed for the treatment of various neoplasms and digestive system disorders, as well as skin and musculoskeletal diseases and urogenital diseases. The active indications for ATX-559 include breast cancer recurrent, colonic cancer, endometrial carcinoma, metastatic colorectal carcinoma, and rectal adenocarcinoma.