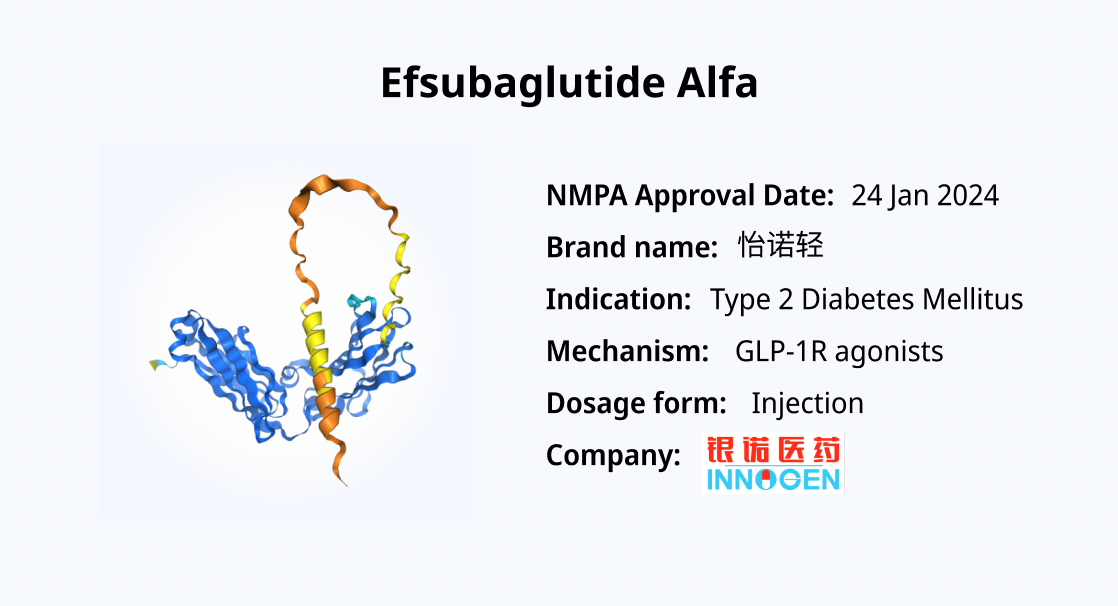
Efsubaglutide Alfa: A Novel GLP-1 Fusion Protein Revolutionizing Type 2 Diabetes
Efsubaglutide Alfa received its first regulatory approval in China on January 24, 2025, from the National Medical Products Administration (NMPA). While Efsubaglutide Alfa is not a traditional antibody, it is a fusion protein based on glucagon-like peptide-1 (GLP-1) and functions as a GLP-1 receptor agonist (GLP-1RA) for the treatment of type 2 diabetes.
Mechanism of Action
GLP-1 is an incretin hormone secreted by intestinal L-cells in response to glucose intake. It plays a crucial role in glucose homeostasis by binding to GLP-1 receptors on pancreatic β-cells, thereby stimulating insulin secretion, suppressing glucagon release, and slowing gastric emptying. These effects contribute to better glycemic control and weight reduction. However, the clinical utility of native GLP-1 is limited due to its extremely short half-life (only a few minutes), as it is rapidly degraded by the enzyme dipeptidyl peptidase-4 (DPP-4).
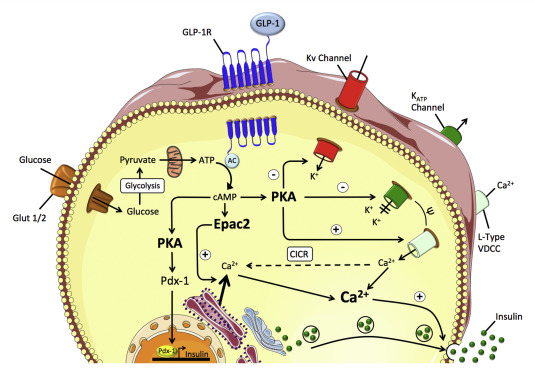
To overcome this limitation, researchers have developed long-acting GLP-1 receptor agonists (GLP-1RAs) such as Efsubaglutide Alfa. This molecule is engineered by fusing GLP-1 with the Fc fragment of IgG2, a strategy that enhances its pharmacokinetic profile. The fusion to IgG2 Fc fragment serves multiple purposes:
·Increases molecular size, reducing renal clearance.
·Enhances structural stability, protecting GLP-1 from enzymatic degradation.
·Minimizes immunogenicity, as IgG2 exhibits lower immunogenic potential compared to other IgG subtypes, contributing to improved safety and tolerability.
Drug Characteristics
Efsubaglutide Alfa incorporates a unique hinge region design, which significantly enhances its binding affinity for the GLP-1 receptor. This high receptor affinity allows for effective receptor activation even at lower drug concentrations, potentially reducing the risk of side effects associated with higher doses.
A key advantage of Efsubaglutide Alfa is its remarkably extended half-life of approximately 204 hours, enabling a once-weekly or biweekly injection regimen. This dosing schedule greatly improves patient convenience and adherence, making it a highly practical option for long-term disease management.
Beyond its primary indication for type 2 diabetes treatment, Efsubaglutide Alfa has also demonstrated cardiovascular benefits and potential efficacy in addressing other metabolic disorders such as obesity. Clinical trials have shown that it significantly promotes weight loss and improves glucose tolerance, making it a valuable therapeutic option for managing chronic metabolic conditions.
Given its prolonged half-life, potent glucose-lowering effects, and additional metabolic benefits, Efsubaglutide Alfa represents not only a powerful addition to existing diabetes therapies but also a promising candidate for broader applications in metabolic disease management.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!
Refrence
1. Müller TD, Finan B, Bloom SR, D'Alessio D, Drucker DJ, Flatt PR, Fritsche A, Gribble F, Grill HJ, Habener JF, Holst JJ, Langhans W, Meier JJ, Nauck MA, Perez-Tilve D, Pocai A, Reimann F, Sandoval DA, Schwartz TW, Seeley RJ, Stemmer K, Tang-Christensen M, Woods SC, DiMarchi RD, Tschöp MH. Glucagon-like peptide 1 (GLP-1). Mol Metab. 2019 Dec;30:72-130. doi: 10.1016/j.molmet.2019.09.010. Epub 2019 Sep 30. PMID: 31767182; PMCID: PMC6812410.